Pemphigus vegetans in a patient with lung cancer
Published Web Location
https://doi.org/10.5070/D3959528svMain Content
Pemphigus vegetans in a patient with lung cancer
Agnieszka B Serwin MD, Ewa Bokiniec MD, and Bozena Chodynicka MD
Dermatology Online Journal 11 (1): 13
Department of Dermatology and Venereology, Medical University of Bialystok, Poland. agabser@amb.edu.pl
Abstract
A 66-year-old woman with a history of lung cancer treated with radiotherapy 2-years prior to admission, was seen for mucosal and skin lesions of 3-month's duration. She had pustules involving intertriginous areas and erosions involving the oral mucosa. Histopathology of skin lesions and the results of direct and indirect immunofluorescence studies were consistent with the Hallopeau-type pemphigus vegetans. Additionally, circulating antibodies against skeletal muscles were detected in patient's serum. The patient was treated with immunosuppressive therapy and had an almost complete remission of skin and mucosal lesions within 1 month; however, the patient developed pneumonia with pyothorax, apparently related to recurrence of lung cancer.
Clinical synopsis
A 66-year-old woman was admitted to the Department in July 2003 for mucosal and cutaneous lesions of 3-month's duration. She had painful erosions in her mouth that made eating and drinking almost impossible; treatment with itraconazole was not successful. After 2 months small pustular lesions appeared in the left submammary region, the right groin, periumbilical area, vulvar and perianal regions, and the occipital region of the scalp. Appproximately 2 weeks prior to admission she developed paronychia of the left great toe with subsequent rapid onycholysis and inflammation of the skin of the toe. The patient had a history of cancer of the right lung (carcinoma planoepitheliale on histopathology) diagnosed in May 2001 and treated with radiotherapy (a total dose of 60 Gy) and with brachytherapy (a total dose of 20 Gy) in 2002.
|
|
| Figure 1 | Figure 2 |
|---|---|
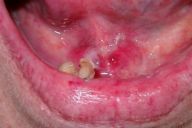
| Deep erosions in the oral mucosa (Fig. 1) | |
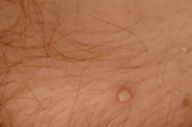
| A newly formed pustula in the pubic area (Fig. 2) | |
On admission she was ill appearing, thin, and pale. Flaccid and easily ruptured pustules evolving into eroded, malodorous, vegetating plaques were present in intertriginous areas. Erosions of the oral mucosa were also present (Fig. 1). Within the first days of hospitalization new pustular lesions developed in the left axilla, under the right breast, and in the pubic area (Fig. 2).
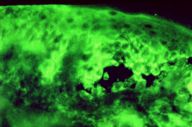
Laboratory examination revealed an erythrocyte sedimentation rate of 128 mm/hour, anemia, thrombocytosis, and hypoalbuminaemia. Other laboratory findings including liver and kidney function tests were normal. A Candida culture from oral mucosa was negative. The histological examination of skin lesions from periumbilical area showed suprabasal acantholysis, intraepidermal microabscesses filled with eosinophils, acanthosis, papillomatosis, and a dermal perivascular infiltrate composed of lymphocytes, plasma cells, and histiocytes. Direct immunofuorescence of perilesional skin revealed intercellular staining of the epidermis with anti-IgG, anti-IgA, anti-IgM, and anti-C3 antibodies (Fig. 3). Circulating pemphigus-type antibodies with the indirect immunofluorescence were detected on monkey esophagus (titer 1:320). In addition, circulating antibodies against skeletal muscle were detected in patient's serum with a titre of 1:160, using monkey heart muscle as a substrate.
The patient was diagnosded with Pemphigus vegetans, Hallopeau type.
After a consultation with the oncologist who did not find evidence of recurrence of malignancy, the patient gave her consent for immunosuppressive therapy and started treatment with prednisone (60 mg/day or 1mg/kg) and cyclophosphamide (100 mg/day). Within 1 month a complete remission of mucosal and cutaneous lesions was achieved. The ESR decreased and the number of thrombocytes returned to normal values. The dose of cyclophosphmide was diminished to 50 mg/day. The immunosuppressive therapy was suddenly ceased in September because the patient developed pneumonia complicated with a lung abscess and pyothorax, despite concomitant wide-spectrum intravenous antibiotherapy. The culture of pleural exsudate showed the growth of Streptococcus anginosus and anaerobic bacteria. In the absence of pemphigus treatment the patient remained asymptomatic for 1 month, then new pustules appeared in previously affected areas and on the oral mucosa. Because of severe respiratory failure the patient's general status is serious.
Comment
Pemphigus vegetans (p. vegetans) was decribed as a variant of pemphigus vulgaris by Neumann in 1876 [1]. Thirteen years later Hallopeau suggested another variant of p. vegetans [2]. Skin lesions in Hallopeau type of p. vegetans typically begin with grouped pustules. The disease is more benign than Neumann type and long remissions are more frequent. On histopathology, it is characterized by the presence of intraepidermal microabscesses in addition to suprabasal acantholysis [3, 4].
The unusual interest of this case is three-fold, concomitance of p. vegetans with a malignancy, the existence of antiskeletal-muscle antibodies in addition to pemphigus antibodies in patient's serum, and a therapeutic complication of pemphigus.
The incidence of neoplasms in patients with pemphigus is from 5 to 12 percent of the total of pemphigus cases and is significantly greater than in controls or than chance expectation [5, 6, 7]. Among Japanese patients, lung cancer is the most commonly associated malignancy [6]. In patients with nonthymic neoplasms, pemphigus may precede or follow the detection of malignancy [5, 7]. As apposed to paraneoplastic pemphigus, preexisting malignancy is more often the case (two-thirds of cases) [8]. When pemphigus follows the development (or detection) of a neoplasm, the time interval is from 1 to 13 months [7]. Our patient had lung cancer diagnosed and treated more than 2 years prior to the onset of pemphigus. The cases of coexistence of p. vegetans and the neoplasms are very rare in the literature. Krain and Bierman [7] described a patient who developed lymphosarcoma 6 years after the diagnosis of p. vegetans that was treated with prednisone (7.5 mg daily) for maintenance therapy. Bastiaens et al. [4] reported a case of p. vegetans (Hallopeau type) with coexisting lung cancer (squamous cell carcinoma on histopathology). Their patient responded well to the pemphigus therapy, underwent successful pneumonectomy, however died suddenly soon afterwards.
It has been suggested that radiotherapy can be a provoking factor for the development of pemphigus in patients with malignancy by inducing a severe immunosuppression, by altering the antigenicity of the keratinocyte surface, or by unmasking epidermal antigens [7, 9]. These new antigenic determinants could induce the production of antibodies by the host or by the tumor. In previously described cases of pemphigus induced by radiotherapy the threshold dose was about 40 Gy (38-100 Gy); the initial site of pemphigus was exclusively limited to that of the irradiation, and the time interval between the radiotherapy and the onset of pemphigus was usually short—several days to several weeks [9]. Taking into consideration the above criteria of location of skin lesions and time interval between the radiotherapy and the onset of pemphigus, a causal relationship between radiotherapy and p. vegetans in our patient seems unlikely.
The presence of a high titer of antiskeletal-muscle antibodies in a patients with pemphigus vulgaris was described only once by McKee and coworkers [10]. That patient had concomitant retroperitoneal tumor (paraganglioma) and, similarly to our patient, had no clinical manifestations of myasthenia gravis. It has been observed that antiskeletal-muscle antibodies can be detected occasionally also in patients with inflammatory or wasting diseases involving skeletal muscles [10]. If our patient is followed for a sufficiently long period of time the clinical symptoms will probably develop.
Because there is no approach that can suppress only desmoglein-autoantibody production, the therapy of pemphigus is based on nonspecific immunosuppression, with corticosterois as a first-line agents. Complications of systemic corticosteroids and immunosuppressive therapy are the major cause of mortality in pemphigus [8].
This case appears to be the third description of concomitance of p. vegetans (Hallopeau type) with internal malignancy; this case is also unusual because of the co-existence of anti-skeletal muscle antibody together with pemphigus antibody in patient's serum. This case also brings up the possible role of radiotherapy as a provoking factor for pemphigus in patients with neoplasms and it underscores the severe side effects of immunosuppressive therapy of pemphigus.
Acknowledgments: The authors express their thanks to Professor Stefania Jablonska and to Doctor Zofia Kolacinska-Strasz (Department of Dermatology, Warsaw School of Medicine) for immunologic and immunopathologic studies.
References
1. Neumann I. Vierteljahresschrift fuer Dermatologie and Syphilis. Arch Dermatol Syphil. 1876; 3: 444-447.2. Hallopeau H. Congres International de Dermatologie et de Syphiligraphie tenu a Paris. Paris: Masson, 1889: 334.
3. Ahmed AR, Blose DA. Pemphigus vegetans. Neumann type and Hallopeau type. Int J Dermatol. 1984 Mar;23(2): 135-141. PubMed
4. Bastiaens MT, Zwan NV, Verschueren GLA, Stoof TJ, Nieboer C. Three cases of pemphigus vegetans: induction by enelapril - association with internal malignancy. Int J Dermatol. 1994 Mar;33(3): 168-171. PubMed
5. Younus J, Ahmed AR. The relationship of pemphigus to neoplasia. J Am Acad Dermatol. 1990 Sep; 23(3 Pt 1): 498-502. PubMed
6. Ogawa H, Sakuma M, Morioka S, Kitamura K, Sasai, Imamura S, Inaba Y. The incidence of internal malignancies in pemphigus and bullous pemphigoid in Japan. J Dermatol Sci. 1995 Mar;9(2): 136-141. PubMed
7. Krain LS, Bierman SM. Pemphigus vulgaris and internal malignancy. Cancer. 1974 Apr;33(4): 1091-1099.PubMed
8. Mimouni D, Anhalt GJ. Pemphigus. Dermatol Therapy. 2002 ;15: 362-368.
9. Delaporte E, Piette F, Bergoend H. Pemphigus vulgaris induced by radiotherapy. Ann Dermatol Venereol. 1991;118 (6-7): 447-451. PubMed
10. McKee PH, McClelland M, Sandford JC. Co-existence of pemphigus, anti-skeletal muscle antibody and a retroperitoneal paraganglioma. Br J Dermatol 1978 Oct;99(4): 441-445. PubMed
© 2005 Dermatology Online Journal