Multiple subcutaneous nodules associate with infection following removal of an infected Groshon vascular catheter tip
Published Web Location
https://doi.org/10.5070/D38dq2r7x2Main Content
Multiple subcutaneous nodules associate with Mycobacterium abscessus infection following removal of an infected Groshon vascular catheter tip
Thomas P Hansen MD, Deba P Sarma MD
Dermatology Online Journal 12 (4): 12
Department of Pathology, Creighton University Medical Center, Omaha. tphansen@creighton.eduAbstract
A 41-year-old man with a history of septic right leg underwent a below-the-knee amputation. One month after amputation and removal of Groshon catheter tip, he developed disseminated subcutaneous abscesses attributed to Mycobacterium abscessus.
Clinical synopsis
A 41-year-old man with a past history of chronic hepatitis-C infection, poly-substance abuse, and psychiatric illnesses had undergone a right below-the-knee amputation for cellulitis. He was admitted to the hospital for evaluation of his psychiatric condition and for substance abuse. In the hospital, he had recurrent fever with night sweats and shaking chills. He also developed several non-erythematous 0.5-2.0 cm subcutaneous nodules on his legs, arms, abdomen, and fingers. A punch biopsy of a leg nodule was obtained for bacterial, mycobacterial, and fungal cultures as well as routine histologic examination. The direct acid-fast stain of the biopsy was negative but the mycobacterial culture was positive for growth at 4 days. The organism was identified as Mycobacterium abscessus. A susceptibility test followed the isolation.
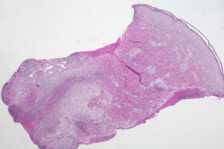 |  |
| Figure 1A | Figure 1B |
|---|---|
| Figure 1A. Low power (2×) view of dermal abscess Figure 1B. High power (40×) view of dermal abscess | |
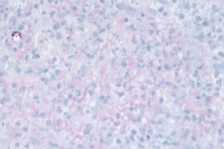 |
| Figure 2 |
|---|
| Figure 2. Acid-fast stain (40×). |
Histological examination showed a dermal abscess with neutrophilic infiltrate and necrosis (Figs. 1A and 1B). An acid-fast stain revealed multiple acid-fast organisms, many arranged in chains, some located within vacuoles (Fig. 2). The case was reviewed by the Department of Dermatopathology of the Armed Forces Institute of Pathology, Washington, D.C. who agreed that the acid-fast positive organisms were consistent with Mycobacterium abscessus.
Some 2 months prior to the skin biopsy, the patient had a positive mycobacterial culture of a Groshon catheter tip typed as Mycobacterium chelonei subspecies abscessus. The patient was started on an antibiotic regimen including clarithromycin, linezolid, cefoxitin, and minocycline.
Comment
Mycobacterium abscessus is a rapidly growing acid-fast mycobacterium. It was formerly known as a subspecies of Mycobacterium chelonae. The organism can typically be found in water, soil, and dust; in addition, M. abscessus has been known to contaminate medications and products, including medical devices [1].
M. abscessus skin and soft tissue infections are rare and are usually associated with immunocompromised hosts or patients on long term corticosteroid treatment. Infection usually occurs by traumatic inoculation into skin or direct contact with preexisting skin lesions. The organism has been documented in skin and soft tissue infections of immunocompromised [2] and immunocompetent hosts [3], keratitis, lymphadenitis, and otomastoiditis [4]. A variety of foreign bodies and other medical devices have been infected including vascular catheters, peritoneal catheters, prosthetic valves, mammoplasty implants [5], and hemodialyzers. The presence of M. abscessus in bronchial washings associated with a contaminated fiberoptic bronchoscope is well documented [6].
Conventional antituberculous medications are not effective against M. abscessus, and treatment may require both surgical debridement and antibiotics [7]. Clarithromycin has been successful in some reports, but resistance to monotherapy with this drug has been documented [8]. Most isolates of M. abscessus are susceptible to amikacin, cefoxitin, imipenem, and clarithromycin. In localized infection, clarithromycin is the drug of choice, with 4-6 month duration of therapy [5]. However, in disseminated disease, combination therapy is recommended to prevent resistance [2].
References
1. Munsiff S, Adams L. 2002 Alert #14: Cluster of Mycobacterium abscessus infections following injections for cosmetic purposes. The City of New York Department of Health, June 30, 2002. www.lapublichealth.org/acd/docs/NYCAlert6-30-02.pdf2. Tebas P, Sultan F, Wallace RJ Jr, Fraser V.. Rapid development of resistance to clarithromycin following monotherapy for disseminated Mycobacterium chelonae infection in a heart transplant patient. Clin Infect Dis. 1995 Feb;20(2):443-4. PubMed
3. Kelley LC, Deering KC, Kaye ET. Cutaneous Mycobacterium chelonei presenting in an immunocompetent host: case report and review of the literature. Cutis. 1995 Nov;56(5):293-5. PubMed
4. TerKonda RP, Levine SC, Duvall AJ 3rd, Giebink GS. Atypical mycobacterial otomastoiditis. Laryngoscope. 1995 Dec;105(12 Pt 1):1275-8. PubMed
5. Mandell G, et al. Principles and practice of infectious disease. 5th ed. New York: Churchill Livingstone; 2000, 2633.
6. Wang HC, Liaw YS, Yang PC, Kuo SH, Luh KT. A pseudoepidemic of Mycobacterium chelonae infection caused by contamination of a fibreoptic bronchoscope suction channel. Eur Respir J. 1995 Aug;8(8):1259-62. PubMed
7. Wallace RJ Jr, Brown BA, Onyi GO. Skin, Soft Tissue, and Bone infections Due to Mycobacterium chelonae, chelonae: Importance of Prior Corticosteroid Therapy, Frequency of Disseminated Infections, and Resistance to Oral Antimicrobials Other than Clarithryomycin. J Infect Dis. 1992 Aug;166(2):405-12. PubMed
8. Wallace RJ Jr, Tanner D, Brennan PJ, Brown BA. Clinical trial of clarithromycin for cutaneous (disseminated) infection due to Mycobacterium chelonae. Ann Intern Med. 1993 Sep 15;119(6):482-6. PubMed
© 2006 Dermatology Online Journal

