Disseminated porokeratosis of Mibelli: A case report
Published Web Location
https://doi.org/10.5070/D33hr1103vMain Content
Disseminated porokeratosis of Mibelli: A case report
Alok Vij MD1, Sean D Doherty MD1, Todd M LeLeux MD2, Sylvia Hsu MD1
Dermatology Online Journal 16 (12): 12
1. Department of Dermatology2. Department of Pathology
Baylor College of Medicine, Houston, Texas. shsu@bcm.edu
Abstract
Porokeratosis is a disorder of clonal hyperproliferation of keratinocytes with several different clinical manifestations. Cutaneous lesions vary in their appearance and distribution. All variants share the distinguishing cornoid lamella on histopathological examination. We present an unusual case of disseminated porokeratosis of Mibelli in an immunocompetent patient.
Case report
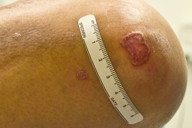
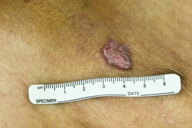
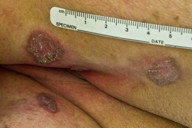
A 75-year-old man presented to our clinic with a 6-year history of progressive pruritic lesions on his left elbow, lower abdomen, groin, buttocks, and thighs. The patient had tried numerous topical steroid creams and ointments, topical and oral antifungal medications, and oral antihistamines with no significant improvement in appearance or symptoms. The patient had no significant past medical history and he had no history of chronic exposure to radiation. The patient had no family history of cutaneous disease. Physical examination revealed multiple large, slightly scaly plaques measuring from 2 - 5 cm located on his left elbow (Figure 1), lower abdomen, groin (Figure 2), buttocks (Figure 4), and thighs. Some of the lesions did appear to have a slightly raised border.
 |  |
| Figure 1 | Figure 2 |
|---|---|
| Figure 1. Scaly 1.5 x 1.0 cm plaque and smaller papule with slightly raised border on the elbow. Figure 2. Scaly 1.5 x 0.8 cm plaque and smaller patches in the groin. | |
 |  |
| Figure 3 | Figure 4 |
|---|---|
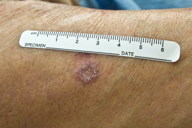
| Figure 3. Annular erythematous plaques on forearm Figure 4. Annular erythematous plaques on buttock | |
Punch biopsy specimens were obtained from two sites, the right groin and the left elbow. Each sample was sent to a different dermatopathology lab for histopathological analysis.
Histopathology
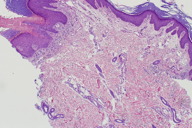 |
| Figure 5 |
|---|
| Figure 5. Histopathology of groin plaque showing prominent cornoid lamellae with underlying loss of the granular layer. (H&E, x100) |
Histopathological examination of both skin biopsy specimens revealed a prominent cornoid lamella with invagination of the epidermis at the site of the cornoid lamella. Interruption of the granular layer below the cornoid lamella was noted (Figure 3). Both specimens were consistent with the diagnosis of porokeratosis.
Treatment and follow-up
The patient was prescribed halobetasol 0.05 percent ointment, which provided significant relief of his pruritus. He was satisfied with this and did not want to pursue more aggressive therapy.
Discussion
Porokeratosis is a group of clonal keratinization disorders characterized by atrophic patches bordered by a distinctly hyperkeratotic cornoid lamella. The five major clinical variants of porokeratosis are: porokeratosis of Mibelli (PM), linear porokeratosis, punctate porokeratosis, disseminated superficial actinic porokeratosis (DSAP), and porokeratosis palmaris et plantaris disseminata (PPPD) [1].
Porokeratosis of Mibelli is characterized by enlarging, brownish papules that may be hyperkeratotic, verrucous, annular, or irregular. The lesions may have central atrophy associated with a peripheral ridge [2]. Linear porokeratosis is characterized by lesions identical to PM distributed along the lines of Blaschko [3] and has an increased risk of tumorigenesis within the lesions [4]. Punctate porokeratosis is defined by discrete, small hyperkeratotic macules on the palms and soles surrounded by a thin, raised margin [5].
Disseminated superficial actinic porokeratosis presents as irregular, thread-like raised rings surrounding discolored skin. The lesions most often present in the 3rd decade of life in patients with chronic sun exposure [6]; a non-actinic form has been described, usually in the context of immunocompromise [7]. Typically, the lesions are greater in number and visibility in areas with greater sun exposure. Superficial skin lesions bounded by a distinct peripheral ridge involving the palms and soles are the hallmark of PPPD [8].
In addition to the varied clinical manifestations of porokeratosis, the disease has various etiologies. Familial variants have been encountered and a corresponding genetic locus has been identified [9]. It is proposed that local or systemic immune dysfunction plays a central role in the development of non-familial porokeratosis. Immunosuppression has been linked to disease occurrence and disease progression [10, 11].
Ionizing radiation of all types has also been linked to the development of DSAP, including natural [12] or artificial [13] ultraviolet radiation and excessive therapeutic photon [14] or electron-beam [15] irradiation. The loss of heterozygosity model has been proposed to explain the link between ionizing radiation and porokeratosis [16].
The plaques observed in our patient most closely resemble the lesions of PM. However, the multifocal distribution of the lesions is unusual. Disseminated PM has been reported following renal [17] and bone marrow transplantation for leukemia, non-Hodgkin lymphoma [18], and myelodysplastic syndrome [19]. However, unlike the previously reported cases, our patient had no history of transplantation or systemic immunosuppression.
Our patient’s condition is also marked by the rare feature of gluteal and crural involvement. PM has previously been described involving the buttocks [20], genitals, and crura [21-23], both separately and in concert [24]. Although individual lesions in those affected with genitogluteal porokeratosis have been reported to spread outside of the gluteal area [24], these reports did not describe secondary lesions arising distant to the primarily affected area. The characteristic finding of porokeratosis on histopathologic examination is the cornoid lamella, which can also be seen in basal cell carcinoma, solar keratosis, and other inflammatory conditions [25]. In other diseases, however, the primary lesion is distinct from that of porokeratosis and other microscopic changes are seen. The microscopic examination of the lesions of our patient did not reveal evidence of a process other than porokeratosis.
Additionally, the age of our patient is atypical. Porokeratosis typically affects patients before the age of 30, unless they subsequently become immunocompromised. Our patient was previously well before his skin lesions presented at the age of 69 and remains healthy at the age of 75 at the time of this report. He had no history of prolonged sun exposure and no family history of similar cutaneous diseases.
Porokeratosis is an uncommon cutaneous disease with a spectrum of presentations, including 5 main types. We report an unusual case of disseminated porokeratosis of Mibelli affecting the arms, legs, abdomen, buttocks, and groin of a healthy 75-year-old man. Although the clinical picture is not classic for PM, histopathological analysis confirmed the diagnosis by the presence of the pathognomonic cornoid lamella.
References
1. Palleshi GM, Tochia D. Porokeratosis of Mibelli and superficial disseminated porokeratosis. J Cutan Pathol. 2008;35(2):253-5. [PubMed]2. Chaudhary RG, Bilimoria F, Katare SK. Large annular plaque with central atrophy on nose. Ind J Dermatol Venereol Leprol. 2009;75(5):552-3. [PubMed]
3. Malhotra SK, Puri KJ, Goyal T, Chahal KS. Linear porokeratosis. Dermatol Online J. 2007; 13(4):15. [PubMed]
4. Happle, R. Cancer proneness of linear porokeratosis may be explained by allelic loss. Dermatol. 1997;195(1):20-5. [PubMed]
5. Lestringant GG, Berge T. Porokeratosis punctata palmaris et plantaris. A new entity? Arch Dermatol. 1989;125(6):816-9. [PubMed]
6. Chernosky ME, Anderson DE. Disseminated superficial actinic porokeratosis. Clinical studies and experimental production of lesions. Arch Dermatol. 1969;99(4):401-7. [PubMed]
7. Auslaender S, Barzilay A, Trau H. Porokeratosis of the skin in an immunosuppressed patient. Harefuah. 1994;127(9):303-5. [PubMed]
8. Jih MH. Porokeratosis plantaris, palmaris, et disseminata. Dermatol Online J. 2003;9(4):34. [PubMed]
9. Xia JH, Yang YF, Deng H, Tang BS, Tang DS, He YG, Xia K, Chen SX, Li YX, Pan Q, Long ZG, Dia HP, Liao XD, Xiao JF, Liu ZR, Lu CY, Yu KP, Deng HX. Identification of a locus for disseminated superficial actinic porokeratosis at chromosome 12q23.2-24.1. J Invest Dermatol. 2000;114(6):1071-4. [PubMed]
10. Kanitakis J, Euvrard S, Faure M, Claudy A. Eur J Dermatol. 1998;8(7):459-65. [PubMed]
11. Mizukawa Y, Shiohara T. Virus-induced immune dysregulation as a triggering factor for the development of drug rashes and autoimmune disease: with emphasis on EB virus, human herpesvirus 6 and hepatitis C virus. J Dermatol Sci. 2000;22(3):169-80. [PubMed]
12. Ibbotson SH. Disseminated superficial porokeratosis: what is the association with ultraviolet radiation? Clin Exp Dermatol. 1996;21(1):48-50. [PubMed]
13. Lederman JS, Sober AJ, Lederman GS. Psoralens and ultraviolet A, immunosuppression, and porokeratosis. J Am Acad Dermatol. 1986;14(2 Pt 1):284-5. [PubMed]
14. James AJ, Clarke LE, Elenitsas R, Katz K. Segmental porokeratosis after radiation therapy for follicular lymphoma. J Am Acad Dermatol. 2008;58(2 Suppl):S49-50. [PubMed]
15. Romani J, Pujol RM, Casanova JM, de Moragas JM. Disseminated superficial porokeratosis developing after electron-beam total skin irradiation for mycosis fungoides. Clin Exp Dermatol. 1996;21(4):310-2 [PubMed]
16. Zhang ZH, Niu ZM, Huang W, Xiang LH, Gu CY, Yang L, Zheng ZZ. Loss of heterozygosity on chromosome 12q in disseminated superficial actinic porokeratosis. J Invest Dermatol. 2007;127(2):482-5. [PubMed]
17. Knoell KA, Patterson JW, Wilson BB. Sudden onset of disseminated porokeratosis of Mibelli in a renal transplant patient. J Am Acad Dermatol. 1999;41(5 Pt 2):830-2. [PubMed]
18. Alexis AF, Busam K, Myskowski PL. Porokeratosis of Mibelli following bone marrow transplantation. Int J Dermatol. 2006 Apr;45(4):361-5. [PubMed]
19. Cha SH, Park HJ, Lee JY, Cho BK. Atypical porokeratosis developing following bone marrow transplantation in a patient with myelodysplastic syndrome. Ann Dermatol. 2010;22(2):206-8. [PubMed]
20. Yong AS, Singh M, Goulding JM, Swale VJ. Follicular porokeratosis of Mibelli on the buttocks. Clin Exp Dermatol. 2009;31(1)43-5. [PubMed]
21. Chen TJ, Chou YC, Chen CH, Kuo TT, Hong HS. Genital porokeratosis: a series of 10 patients and review of the literature. Br J Dermatol. 2006;155:325-9. [PubMed]
22. Laino L, Pala S, Innocenzi D, Accappaticcio G, van Steensel MAM. Genital porokeratosis. Eur J Dermatol. 2004 May-Jun;14(3):190-2. [PubMed]
23. Trcka J, Pettke-Rank CV, Brocker E-B, Hamm H. Genitoanocrural porokeratosis following chronic exposure to benzene. Clin Exp Dermatol. 1998;23(1):28-31. [PubMed]
24. Huang SL, Liu YH, Chen W. Genitogluteal porokeratosis. J Eur Acad Dermatol Venereol. 2006 Aug;20(7):899-900. [PubMed]
25. Wade TR, Ackerman AB. Cornoid lamellation. A histologic reaction pattern. Am J Dermatopathol. 1980;2(1):5-15. [PubMed]
© 2010 Dermatology Online Journal

