Lichenoid drug eruption
Published Web Location
https://doi.org/10.5070/D335c1029xMain Content
Lichenoid drug eruption
Jeremy Brauer MD, Henry J Votava MD, Shane Meehan MD, Nicholas A Soter MD
Dermatology Online Journal 15 (8): 13
Department of Dermatology, New York UniversityAbstract
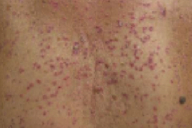
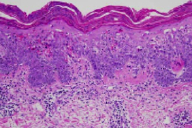
A 78-year-old man presented with an eight-month history of folliculocentric, pink, hyperkeratotic papules and plaques with thick white scale that involved the entire body, with confluence on the buttocks and genitalia. A biopsy specimen demonstrated superficial and focal, mild perivascular and perifollicular, band-like lymphocytic infiltrate and eosinophils. There were lymphocytes extending to the dermo-epidermal junction with vacuolar changes. A diagnosis of lichenoid drug eruption secondary to a proton-pump inhibitor was made. To the best of our knowledge, only one other case of lichenoid drug eruption secondary to a proton-pump inhibitor has been reported.
 |  |
| Figure 1 | Figure 2 |
|---|---|
History
A 78-year-old man initially presented to the Charles C. Harris Skin and Cancer Pavilion in October, 2008, for evaluation of an eight-month history of a generalized eruption. He first noticed white bumps on his arms that progressively spread to involve the trunk, legs, and, most recently, the face, particularly under the chin and on the ears. There was occasional pruritus; however, he denied tenderness.
The patient had been evaluated by several dermatologists prior to his referral to the Charles C. Harris Skin and Cancer Pavillion and underwent multiple biopsies.
At the time of his presentation, he was not applying or taking any medications for his condition. Previous therapies included trimethoprim/sulfamethoxazole, over-the-counter lotions, permethrin 5 percent cream, triamcinolone 0.1 percent lotion, and triamcinolone 0.1 percent ointment without benefit. Levocetirizine was associated with relief of pruritus.
The patient reported that at the onset of the initial eruption, he had recently changed medications from lansoprazole to a generic omeprazole. Additionally, he had traveled to Greece the month before. He denied initiation of new lotions, colognes, detergents, soaps, and shampoos. He lives with his sister, who had not experienced similar symptoms.
Past medical history included hypertension, gastroesophageal reflux disorder, and benign prostatic hypertrophy. Medications included nifedical, terazosin, and oxybutinin. He had no known drug allergies and no family history of skin disease.
Physical Examination
On the head, neck, chest, back, abdomen, genitalia, buttocks, and the upper and lower extremities were folliculocentric, pink, hyperkeratotic papules and plaques with thick white scale. There was confluence on the buttocks and genitalia.
Laboratory data
None.
Histopathology
(Outside specimens): There is a lichenoid interface dermatitis with a superficial, perivascular dermatitis and eosinophils. (NYU specimens): There is a superficial and focal, mild perivascular and perifollicular band-like lymphocytic infiltrate. Lymphocytes extend to the dermo-epidermal junction with vacuolar changes. Numerous necrotic keratinocytes are present throughout the epidermis, with extravasation of erythrocytes into the epidermis and pallor of superficial layers. There is near confluent parakeratosis with neutrophils.
Comment
Lichenoid drug eruptions (LDE) have been associated with a wide variety of medications. As new agents continue to be discovered and prescribed, the list of offenders continues to grow steadily. Inducers include but are not limited to gold, antimalarial agents, angiotensin-converting-enzyme inhibitors, nonsteroidal anti-inflammatory agents, thiazide diuretics, penicillamine, dental amalgams, sulfasalazine, beta blockers, Viagra, and proton-pump inhibitors [1, 2, 3, 4, 5].
Clinically these eruptions are often similar, if not identical, to those of lichen planus (LP) and present as a generalized distribution of papules or plaques, commonly on sun-exposed areas, such as the extensor aspects of the extremities and dorsal aspects of the hands. Compared to the lesions of LP, those of LDE often may be larger in size, less monomorphic, and more likely to be eczematous and associated with desquamation and crust [3, 6]. Furthermore these lesions typically spare the classic sites of lichen planus, such as the flexor surfaces, mucous membranes, and genitalia, and often do not have Wickham's striae [3, 6].
Histopathologically, the two entities possess similar findings, and therefore it is not possible to differentiate between them on histopathologic criteria alone. However, several findings are more typical of specimens of LDE and include focal parakeratosis, presence of eosinophils and plasma cells, and a deeper perivascular and periadnexal infiltrate [3, 5].
Most patients typically recall an interval between the initiation of the offending agent and the first appearance of cutaneous manifestations. A latent period as long as an average of twelve months has been reported between the beginning of treatment and the onset of LDE [4]. This latent period appears to be dependent upon the class of drug, the dosage, the individual reaction, and presence or absence of additional medications. It has been demonstrated that LDE secondary to penicillamine therapy may not appear for two months to three years [7], the latent period is three to six months for angiotension-converting-enzyme inhibitors [8], and four to six weeks for quinacrine [9]. This period may be shortened if the individual has had previous exposure to the agent.
Treatment includes removal of the offending agent, and topical glucocorticoids [2]. The length of time to resolution after discontinuation also has a wide range and has been reported to range from a couple of weeks to a year [4].
To the best of our knowledge at the time of this report, there has been only one report in the literature of an elderly man, who developed a recurrent, lichenoid eruption after treatment with omeprazole, lansoprazole, and pantoprazole [2]. The patient had been on the medication for a period of six months before developing a pruritic, annular, scaly, erythematous eruption on his trunk and upper and lower extremities that ultimately resolved with discontinuation of this class of medication.
Additional adverse omeprazole-induced dermatologic manifestations published as case reports include urticaria, lichen planus, dermatomyositis, pityriasis rosea-like eruption, and erythema nodosum [10, 11, 12, 13, 14].
References
1. DeRossi SS, Ciarrocca KN. Lichen planus, lichenoid drug reactions, and lichenoid mucositis. Dent Clin N Am 2005;49: 77 [PubMed]2. Bong JL, et al. Lichenoid drug eruption with proton pump inhibitors. Br Med J 2000; 320:283 [PubMed]
3. Antiga E, et al. A case of lichenoid drug eruption associated with sildenafil citratus. J Dermatol 2005;32:972 [PubMed]
4. Halevy S, Shai A. lichenoid drug eruption. J Am Acad Dermatol 1993;29:249 [PubMed]
5. Van den Haute V, et al. Histopathological discriminant criteria between lichenoid drug eruptions and idiopathic lichen planus: retrospective study on selected samples. Dermatologica 1989;179:10 [PubMed]
6. Bork K. Lichenoid eruptions. In Bork K, ed. Cutaneous Side Effects of Drugs. Philadephia: WB Saunders, 1988:170
7. Powell FC, et al. Primary biliary cirrhosis and lichen planus. J Am Acad Dermatol 1983;9:540-5. [PubMed]
8. Rotstein E, Rotstein H. Drug eruptions with lichenoid histology produced by captopril. Australas J Dermatol 1989;30:9 [PubMed]
9. Bauer F. Quinacrine hydrochloride drug eruption (tropical lichenoid dermatitis). J Am Acad Dermatol 1981;4:239 [PubMed]
10. Delpre G, et al. Urticaria during triple therapy for Helicobacter pylori infection: clinical implications. Digestive Diseases Sciences 1997;42:728 [PubMed]
11. Gruppo Italiano Studi Epidemiologici in Dermatologia. Cutaneous reactions to alimentary tract medications: results of a seven-year surveillance program and review of the literature. Dermatology 1996; 193: 11 [PubMed]
12. Pan Y, et al. Omeprazole-induced dermatomyositis. Br J Dermatol 2006;154:557 [PubMed]
13. Buckley C. Pityriasis rosea-like eruption in a patient receiving omeprazole. Br J Dermatol 1996; 135:660 [PubMed]
14. Ricci RM, Deering KC. Erythema nodosum caused by omeprazole. Cutis 1996;57:434 [PubMed]
© 2009 Dermatology Online Journal

