Alopecia areata universalis during off-label treatment with Infliximab in a patient with Behçet disease
Published Web Location
https://doi.org/10.5070/D331c9h7fxMain Content
Letter: Alopecia areata universalis during off-label treatment with Infliximab in a patient with Behçet disease
E Beccastrini1, D Squatrito1, G Emmi1, P Fabbri2, L Emmi1
Dermatology Online Journal 16 (9): 15
1. Allergology and Clinical Immunology, Department of Biomedicine, University of Florence, Florence, Italy2. Department of Dermatological Sciences, University of Florence, Italy
Abstract
Infliximab, a chimeric monoclonal anti-TNF-alfa agent used to treat autoimmune diseases, has shown a paradoxical side effect in the development of autoimmunity. We describe a case of alopecia areata universalis associated with infliximab treatment in a patient with Behçet disease. This case suggests a complex and contradictory role of TNF-α in the pathogenesis of alopecia areata.
Introduction
Six cases of alopecia areata (AA) in patients treated with infliximab have been reported in the literature (Table 1) [1-7]. We describe the first case of AA universalis that developed during infliximab treatment.
Clinical presentation
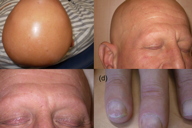
The patient is a 41-year-old man with Behçet disease (BD), whose diagnosis was performed on the basis of recurrent orogenital ulcerations, severe uveitis, asymmetrical arthrithis involving knees, and HLA-B51 allele positivity. The orogenital ulcerations and the ocular manifestations have been successfully treated with prednisolone and colchicine. After 6 months, a relapse with active eye disease was treated with corticosteroids and cyclosporine A (CSA), giving a rapid improvement. The withdrawl of glucocorticoids and maintaining CSA, however, allowed a relapse of the disease and treatment with azathioprine was attempted. Unfortunately, worsening of the visual impairment was observed. Therefore, infliximab was administred at a dose of 5 mg/kg, through intravenous infusion at weeks 0, 2, 6, and 8, then every 8 weeks. In a few weeks there was a marked improvement of the orogenital ulceration and of the posterior uveitis. Treatment with infliximab alone was continued with no relapse nor evidence of disease recurrence. After one year the patient suddenly developed multifocal, smooth patches of hair loss with short broken hairs at the periphery and nail dystrophy. The hair loss progressed resulting within 6 months in a typical form of AA universalis with nail abnormalities (Figure 1). There were neither discernable psychosomatic triggering factors, nor familiar or personal history of hair loss. The patient’s serum was negative for evidence of antinuclear antibodies. Infliximab treatment was discontinued and the patient was treated with clobetasol propionate 0.05 percent and topical minoxidil. Three months after infliximab discontinuation, a partial hair regrowth was observed.
Discussion
Alopecia areata is an autoimmune inflammatory disease, in which T lymphocytes play a central pathogenetic role. Although the exact pathophysiology of AA remains unknown, it is postulated that CD4+ and CD8+ T cells reactive to hair bulb autoantigens induce the autoimmune process leading to non-scarring hair loss. Alopecia areata is frequently associated with other autoimmune diseases such as vitiligo, Hashimoto disease, rheumatoid arthritis, or insulin-dependent diabetes, but association with BD or vasculitis has not been described. The pathogenesis of AA was traditionally thought Th1-mediated [8].
This case suggests a complex and contradictory role of TNF-α in the pathogenesis of AA. Increased IL-1β, IFN-γ, IL-2, and TNF-α expression has been demonstrated in AA lesions and in vitro studies have shown that TNF-α and IL-1β were potent inhibitors of hair follicle growth. Therefore, TNF-α was considered to have an important role in the AA hair loss [9].
Strober et al, however, have shown no efficacy of anti-TNF-α agents in the treatment of AA [10]. Moreover, although anti-TNF-α agents are expected to treat autoimmune-related processes similar to AA, these drugs have been associated with the development of other autoimmune diseases [11]. Ettefagh et al proposed that TNF-α is not necessary for the development of inflammation in AA and that anti-TNF-α agents could cause a dysregulation of cytokines leading to inflammation [2].
The role of anti-TNF-α agents in the pathogenesis of autoimmune diseases and, more specifically, of AA remains mainly elusive. Future work will be required to distinguish exactly which factors link anti-TNF-α agents with the immune dysregulation that induces AA.
References
1. Ferran M, Calvet J, Almiral M, Pujol RM, Maymó J. Alopecia Areata as another immune-mediated disease developed in patients treated with tumour necrosis factor-α blocker agents. J Eur Acad Dermatol Venereol 2010 Jun; 24 [Epub ahead of print]. [PubMed]2. Ettefagh L, Nedorost L, Mirmirani P. Alopecia areata in a patient using Infliximab: new insights into the role of Tumor necrosis factor on human hair follicles. Arch Dermatol 2004 Aug; 140(8):101. [PubMed]
3. Tosti A, Pazzaglia M, Starace M, Bellavista S, Vincenzi C, Tonelli G. Alopecia areata during treatment with biologic agents. Arch Dermatol 2006 Dec; 142(12):1653-4. [PubMed]
4. Nakagomi D, Harada K, Yagasaki A, Kawamura T, Shibagaki N, Shimada S. Psoriasiform eruption associated with alopecia areata during Infliximab therapy. Clin Exp Dermatol 2009 Dec; 34(8):923-4. [PubMed]
5. Fabre C, Dereure O. Worsening of alopecia areata and de novo occurrence of multiple halo nevi in a patient receiving infliximab. Dermatology 2008; 216(2):185-6. [PubMed]
6. Medkour F, Babai S, Chanteloup E, Buffard V, Delchier JC, Le-Louet H. Development of diffuse psoriasis with alopecia during treatment of Chron’s disease with Infliximab. Gastroenterol Clin Biol 2010 Feb; 34(2):140-1. [PubMed]
7. Hernàndez MV, Noguès S, Ruiz-Esquide V, Alsina M, Cañete JD, Sanmartí R. Development of alopecia areata after biological therapy with TNF-alpha blockers: description of a case and review of the literature. Clin Exp Rheumatol 2009 Sep-Oct; 27(5):892-3. [PubMed]
8. Katagiri K, Arakawa S, Hatano Y. In vivo levels of IL-4, IL-10, TGF-β1 and IFN-γ mRNA of the peripheral blood mononuclear cells in patients with alopecia areata in comparison to those in patients with atopic dermatitis. Arch Dermatol Res 2007 Jan; 298(8):397-401. [PubMed]
9. Hoffmann R, Eicheler W, Huth A, Wenzel E, Happle R. Cytokines and growth factors influence hair growth in vitro. Possible implications for the pathogenesis and treatment of alopecia areata. Arch Dermatol Res 1996 Mar; 288(3):153-6. [PubMed]
10. Strober BE, Siu K, Alexis AF, Kim G, Washenik K, Sinha A, Shupack JL. Etanercept does not effectively treat moderate to severe alopecia areata: an open-label study. J Am Acad Dermatol 2005 Jun; 52(6):1082-4. [PubMed]
11. De Bandt M, Sibilia J, Le Loët X, Prouzeau S, Fautrel B, Marcelli C, Boucquillard E, Siame JL, Mariette X; Club Rhumatismes et Inflammation. Systemic lupus erythematosus induced by anti-tumour necrosis factor alpha therapy: a French national survey. Arthritis Res Ther 2005; 7(3):R545-51. [PubMed]
© 2010 Dermatology Online Journal