Bilateral disseminated herpes zoster in an immunocompetent host
Main Content
Bilateral disseminated herpes zoster in an immunocompetent host
Yumiko Takaoka MD, Yoshiki Miyachi MD, Yoshiaki Yoshikawa MD, Miki Tanioka MD, Akihiro Fujisawa MD, Yuichiro Endo MD
Dermatology Online Journal 19 (2): 13
Kyoto University Graduate School of Medicine, JapanAbstract
Herein we report a rare case of disseminated herpes zoster(HZ) infection involving two widely separated bilateral dermatomes in an immunocompetent host. HZ involving two widely separated areas simultaneously is referred to as HZ duplex bilateralis. It is very rare, with an incidence of less than 0.1 percent of all HZ cases, and usually develops in immunocompromised patients.
Varicella zoster virus (VZV) remains dormant in sensory dorsal root ganglia following primary infection. Herpes zoster (HZ) is caused by reactivation of the dormant virus. Trigger mechanisms include trauma, stress, aging, cancer, and other immunosuppressive conditions, including those related to therapeutic agents [1]. HZ usually affects the left or right dermatome in half of the body [2], but sometimes affects more than one dermatome, particularly in immunocompromised hosts. This condition is referred to as multidermatomal HZ and usually involves dermatomes that are contiguous and unilateral. Herein, we report a rare case of disseminated HZ infection involving two widely separated bilateral dermatomes in an immunocompetent host.
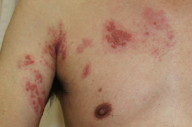
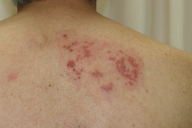
A 61-year-old man presented with diffuse edematous erythema and vesicles on the right side of the chest to the back (right Th2 to Th3) and left arm (left C5 to Th1) (Figures 1 and 2). Two days earlier, he had felt superficial pain and itch in these regions. He had no history of chronic systemic illness and took no medication, including immunosuppressive agents. His family history was unremarkable and there was no consanguinity. He had not had contact with a child with VZV infection. Physical examinations revealed no significant signs other than the cutaneous findings. Body temperature (axillary) was 37.7°C. He had slight headache, but no nausea.
Laboratory tests revealed hemoglobin 13.6 g/dl, red blood cell count 428×104/μL, white blood cell count 5,400/μL (neutrophils 37.4%, lymphocytes 52.2%, monocytes 7.8%, eosinophils 2.2%, basophils 0.4%), and platelet count 187,000/μL. Blood urea nitrogen, serum protein, albumin, and globulin were all normal. Tzanck smear tests from a few vesicles showed giant multinucleated cells. Based on these results, the skin lesions were diagnosed as disseminated HZ infection involving bilateral dermatomes. Treatment was initiated with oral valacyclovir hydrochloride 1 g every 8 h, 3 times daily, for 7 days. Pain was treated by loxoprofen sodium 180 mg/day and gabapentin 200 mg/day. Vitamin B12 1500 μg/day was administered for supportive care. With this treatment, the vesicles diminished and the patient had an uneventful recovery.
Herpes zoster, involving two widely separated areas simultaneously is referred to as HZ duplex bilateralis. It is very rare, with an incidence of less than 0.1 percent of all HZ cases and usually develops in immunocompromised patients. Detection of VZV DNA in trigeminal, cervical, thoracic, lumbar, and sacral dorsal root ganglia in autopsy studies [3, 4] has led to the suggestion that VZV remains latent in most sensory dorsal root ganglia. The common occurrence of viral reactivation on only one side of the body may be related to the VZV genome load distribution in different dorsal root ganglia, with the highest viral genome load leading to clinical HZ [5]. Concurrent VZV-specific immunoboosting induced by HZ eruption probably avoids clinical manifestation of subsequent reactivations. VZV may be reactivated in multiple dorsal root ganglia only when the virus accidentally escapes from cellular immunity in a healthy person, but is more common in an immunosuppressed host.
In our case, reactivation of the latent virus infection seemed to have taken place in the latently infected right and left ganglia of the spinal cord. Although patients with impaired cellular immunity can develop diverse fatal systemic manifestations such as pneumonia, gastroenteritis, and encephalitis, this patient had a normal immune response and seemed to be free of HZ-associated serious complications.
References
1. David E. Elder, Rosalie Elenitsas, George F. Murphy, et al: Lever’s Histopathology of the skin, 10th ed, Lippincott Wiliams & Wilkins, Philadelphia, 643-644, 20092. Gershon AA, Gershon MD, Breuer J, Levin, Oaklander AL, Griffiths PD. Advances in the understanding of the pathogenesis and epidemiology of herpes zoster. J Clin Virol. 2010 May; 48 Suppl 1:S2-7. [PubMed]
3. Gilden DH, Vafai A, Shtram Y, Becker Y, Devlin M, Wellishi M. Varicella-zoster virus DNA in human sensory ganglia. Nature. 1983 Dec 1-7; 306(5942): 478-480. [PubMed]
4. Kennedy PG, Cohrs RJ. Varicella-zoster virus human ganglionic latency: a current summary. J Neurovirol. 2010 Nov; 16(6): 411-418. [PubMed]
5. Pevenstein SR, Williams RK, McChesney D, Mont EK, Smialek JE, Straus SE. Quantitation of latent varicellazoster virus and herpes simplex virus genomes in human trigeminal ganglia. J Virol, 1999 Dec; 73(12):10514-10518. [PubMed]
© 2013 Dermatology Online Journal