Invasive squamous-cell carcinoma and arsenical keratoses
Published Web Location
https://doi.org/10.5070/D36d71r5d9Main Content
Invasive squamous-cell carcinoma and arsenical keratoses
Sarina B Elmariah MD PhD, Robert Anolik MD, Ruth F Walters MD, Karla Rosenman MD, Miriam K Pomeranz MD, Miguel R Sanchez MD
Dermatology Online Journal 14 (10): 24
Department of Dermatology, New York UniversityAbstract
A 42-year-old man presented with a six-month history of a slowly-enlarging ulcer on his right sole, a 30-year history of altered pigmentation of the trunk and extremities, and hyperkeratotic papules of the palms and soles. Histopathologic examination showed an invasive squamous-cell carcinoma of the right sole and hyperkeratosis with keratinocyte atypia of the left finger and left lateral foot. The clinical and histopathologic findings are consistent with chronic arsenicism, which most commonly occurs in the setting of drinking contaminated water or after occupational exposure. Evaluation should include a physical examination, basic laboratory work-up, and measurement of a 24-hour urine arsenic concentration. Vigilant surveillance for the development of cutaneous malignancies is required. Oral retinoids may be helpful in reducing hyperkeratosis secondary to chronic arsenicism.
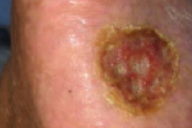 | 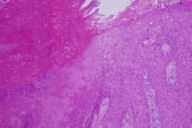 |
| Figure 1 | Figure 2 |
|---|
History
A 42-year-old man presented to the Dermatology Clinic at Bellevue Hospital Center in December, 2007, with a six-month history of a painful, slowly enlarging ulcer on his right sole. The ulcer did not respond to initial treatment with Aquacel, PolyMem, Duoderm, and Regranex gel. A biopsy of the right sole ulcer was performed in January, 2008. The patient was also noted to have mottled pigmentation of the trunk, arms, and legs as well as hyperkeratotic papules and plaques with scale of the palms and soles. These lesions had been present for 30 years. Additional biopsies of the left lateral foot and the left fourth digit were performed in February, 2008. He denied systemic complaints. Review of systems was negative.
Medical history was noncontributory. He did not take medications and denied drug allergies. Family and social histories disclosed that the patient was born in a rural village in Ecuador and had immigrated to the United States 20 years ago. Throughout his childhood in Ecuador, he and other members of his village drank water from a local well. The patient's sister and neighboring villagers had similar pigmentation and hyperkeratotic lesions of the palms and soles.
Physical Examination
On the chest, axillae, arms, and legs, faint, hyperpigmented patches with superimposed guttate hypopigmentation were noted. On the palms and soles, numerous, yellow, firm, hyperkeratotic papules and plaques with surrounding collarettes of scale were present. On the right sole, an irregularly-shaped, 2.5-cm x 2.0-cm, shallow ulcer with a red granular base and macerated white borders was present. There was no lymphadenopathy or hepatosplenomegaly.
Lab
A complete blood count showed a mild thrombocytopenia with platelets of 110 x 109/L but was otherwise normal. Erythrocyte sedimentation rate was 1 mm/hr and C reactive protein was 0.39 mg/L. Comprehensive metabolic and hepatic function panels were normal except for an alkaline phosphatase of 109 U/L. Serum arsenic level was < 3 mcg/L and 24-hour urine arsenic level was 60 mcg/L. A radiograph of the right foot in February, 2008, showed a mild hallux valgus deformity with minimal metatarsophalangeal joint osteoarthritis without osseus destruction. A computed tomography scan of the chest, abdomen, and pelvis in February, 2008, showed a few, small, shotty inguinal lymph nodes bilaterally without metastatic foci or osseus involvement.
Histopathology
There is epidermal hyperplasia with full-thickness keratinocytic atypia in addition to irregularly-shaped nests of keratinocytes within the reticular dermis. There is adjacent acanthosis with mild keratinocytic atypia of basilar keratinocytes.
Comment
Arsenic is a naturally occurring metalloid found in the earth's crust and within numerous ores [1]. Human exposure occurs via contaminated drinking water, agricultural and industrial exposures, and medicinal applications. Chronic arsenicism results in cutaneous and systemic toxicity, which leads to the development of benign and malignant tumors.
Chronic arsenic exposure due to the ingestion of naturally-contaminated water is a growing public health concern and affects populations in Bangladesh, India, Taiwan, Japan, Mexico, Chile, Argentina, Canada, and the United States [2-5]. Groundwater contamination due to leaching of arsenic from rocks and soil frequently results in concentrations above 50 mcg/L, which is associated with carcinogenesis in humans [2]. Occupational exposure via inhalation of arsenic-containing vapors occurs during smelting, mining, refining, electroplating, and manufacturing of decorative-glass, pesticides, gallium arsenide computer microchips, and pressure-treated wood [4-6]. Therapeutic arsenic compounds, such as liquor potassii arsenitis (Fowler's solution) and arsenic pills with opium or pepper (Asiatic pills), remain in use in China and India [4].
The cutaneous manifestations of chronic arsenicism have an insidious onset. Hyperpigmentation of the axillae, groin, areolae, and acral surfaces and guttate hypopigmentation are the earliest manifestations, which occur ten to 20 years after the onset of exposure [4, 7]. Hyperkeratosis and scale of the palms and soles, eczematous patches of the extremities, and alopecia subsequently may develop. Bowen's disease, squamous-cell carcinomas, and basal-cell carcinomas often arise in pre-existing arsenical keratoses and may be aggressive, with metastasis occurring in one-third of squamous-cell carcinomas [7, 8, 9, 10]. Chronic arsenic exposure also may lead to other systemic findings, which include progressive sensorimotor neuropathy, hypertension, vascular disease, diabetes mellitus, conjunctivitis, bone marrow hypoplasia, gastrointestinal disturbances, and malignant neoplasms of the lung, bladder, and liver [4, 8, 9, 10].
The precise mechanisms by which arsenic induces carcinogenesis in human tissues remain unknown. Arsenic has been shown to cause uncoupling of oxidative phosphorylation, which leads to the production of reactive oxygen species and increased cellular stress in vitro [7, 11]. Arsenic also may be directly genotoxic such that it can induce chromosomal abnormalities, gene amplification, and chromatid exchange. It may also inhibit DNA repair synthesis and modulate the expression of p53 and activating protein-1 [11-13]. A recent study showed that polymorphisms in a nucleotide excision repair pathway gene, ERCC2, are associated with increased susceptibility to arsenic-induced genotoxic effects and formation of premalignant keratoses [14].
Histopathologic findings of arsenical keratoses include marked hyperkeratosis with scattered parakeratosis and mild-to-moderate cytologic atypia of keratinocytes. A perivascular, lymphocytic infiltrate and dermal connective-tissue degeneration may be present. Clinical history often is required to distinguish between actinic and arsenical keratoses [4].
Laboratory tests should include a complete blood count and renal and liver function tests. Arsenic is rapidly cleared from the blood, so measurement of arsenic on a 24-hour or spot urine collection should be performed. A concentration greater than or equal to 50 mcg/L or 100 mcg of arsenic per gram creatinine in the absence of recent fish or shellfish intake strongly suggests arsenic poisoning [5, 7, 10]. If there are signs or symptoms of peripheral neuropathy, nerve conduction tests should be considered.
Treatment of the cutaneous manifestations of chronic arsenicism is limited. Emphasis is placed on the elimination of further exposure to arsenic and aggressive surveillance for the development of skin cancers. Oral retinoids have been reported to reduce hyperkeratosis, scale, and the risk of cutaneous malignancy formation [15, 16, 17]. Surgical excision, cryotherapy, curettage, and topical chemotherapy have been used successfully to manage cutaneous malignant conditions that arise in the setting of chronic arsenicism [4, 18]. Few studies support the use of chelation therapy in chronic arsenic toxicity.
References
1. Duker AA, et al. Arsenic geochemistry and health. Environ Int 2005; 31: 631 PubMed2. McDonald C, et al. Risk of arsenic-related skin lesions in Bangladeshi villages at relatively low exposure: a report from Gonoshasthaya Kendra. Bull World Health Organ 2007; 85: 668 PubMed
3. International Agency on Research in Cancer Monograph on the Evaluation of Carcinogenic Risk to Humans, Some Drinking Water Disinfectants and Contaminants, including Arsenic. Lyon, France, 2004; 84: 1 PubMed
4. Smith ML. Environmental and sports-related skin diseases. In: Bolognia, JL, et al., eds. Dermatology. 2nd ed. London: Mosby, 2008: 1364
5. Ford, MD. Arsenic. In: Goldfrank, L et al., eds. Goldfrank's Toxicological Emergenices. New York: McGraw-Hill, 2002: 1183
6. Aposhian H. Biochemical toxicology of arsenic. Rev Biochem Toxicol 1989; 10: 265
7. Tchounwou PB, et al. Arsenic toxicity, mutagenesis, carcinogenesis - a health risk assessment and management approach. Mol Cell Biochem 2004; 255: 47 PubMed
8. Windebank, AJ. Arsenic. In: Spencer, PS et al. eds. Experimental and Clinical Neurotoxicology. New York: Oxford University Press, 2000: 203
9. Chiou HY, et al. Incidence of internal cancers and ingested inorganic arsenic: a seven-year follow-up study in Taiwan. Cancer Res 1995; 55: 1296 PubMed
10. Lauwerys RR, et al. Industrial Chemical Exposure - Guidelines for Biological Monitoring, Boca Raton: Lewis Publishers, 2000; 1
11. Liu SX, et al. Induction of oxyradicals by arsenic: implication for mechanism of genotoxicity. Proc Natl Acad Sci USA 2000; 98:1643 PubMed
12. Ghosh P, et al. Cytogenetic damage and genetic variants in the individuals susceptible to arsenic-induced cancer through drinking water. Int J Cancer 2006; 118: 2470 PubMed
13. De Chaudhuri S, et al. Arsenic-induced health effects and genetic damage in keratotic individuals: involvement of p53 arginine variant and chromosomal aberrations in arsenic susceptibility. Mutat Res 2007; 1 PubMed
14. Banerjee M et al. Polymorphisms in the ERCC2 codon 751 is associated with arsenic- induced permalignant hyperkeratosis and significant chromosome aberrations. Carcinogenesis 2007; 28: 672 PubMed
15. Son SB, et al. Successful treatment of palmoplantar arsenical keratosis with a combination of keratolytics and low-dose acitretin. Clin Exp Dermatol 2008; 33: 202 PubMed
16. Yerebakan O, et al. Treatment of arsenical keratosis and Bowen's disease with acitretin. Int J Dermatol 2002; 41: 84 PubMed
17. Sharma SC, et al. Treatment of arsenical keratosis with etretinate. Acta Derm Venereol 1983; 63: 449 PubMed
18. Boonchai W. Treatment of precancerous and cancerous lesions of chronic arsenicism with 5% imiquimod cream. Arch Dermatol 2006; 142: 531 PubMed
© 2008 Dermatology Online Journal

