DRESS syndrome associated with raltegravir
Published Web Location
https://doi.org/10.5070/D39x26t1qnMain Content
Letter: DRESS syndrome associated with raltegravir
Kevin S Zhang MD, Gunjan M Modi MD, Sylvia Hsu MD
Dermatology Online Journal 17 (8): 14
Department of Dermatology, Baylor College of Medicine, Houston, Texas. shsu@bcm.eduAbstract
Drug reaction with eosinophilia and systemic symptoms (DRESS) syndrome or drug-induced hypersensitivity is a potentially life-threatening drug hypersensitivity syndrome most commonly associated with anticonvulsants, allopurinol, long-acting sulfonamides, dapsone, and minocycline. In the setting of HIV infection, the antiretroviral medicines abacavir, nevirapine, and efavirenz have all shown well-documented associations with DRESS syndrome. There has only been one case (in a poster presentation) of this syndrome in a patient who was taking raltegravir.
Introduction
Drug reaction with eosinophilia and systemic symptoms (DRESS) syndrome or drug-induced hypersensitivity is a potentially life-threatening drug hypersensitivity syndrome most commonly associated with anticonvulsants, allopurinol, long-acting sulfonamides, dapsone, and minocycline [1, 2]. In the setting of HIV infection, the antiretroviral medicines abacavir, nevirapine, and efavirenz have all shown well-documented associations with DRESS syndrome [3, 4, 5]. There has only been one case (in a poster presentation) of this syndrome in a patient who was taking raltegravir [6].
Case report
A 64-year-old woman was diagnosed with AIDS in 2002 and had been previously taking abacavir, lamivudine, and zidovudine for 8 years. She also had a history of hypertension and hyperlipidemia for which she was taking hydrochlorothiazide, verapamil, and atorvastatin (each for the past 8 years), as well as verapamil (for the past 12 months). Because of viral resistance, her anti-retroviral therapy was changed to raltegravir, ritonavir, and darunavir in March 2011. By April, her CD4 count was 522 and her viral load was undetectable. Six weeks after initiation of the new anti-retroviral regimen, she presented to the emergency room with a 9-day history of worsening generalized morbilliform exanthem, pruritus, pronounced facial erythema and edema (Figure 1), and diffuse lymphadenopathy, despite stopping her HIV medicines within 1 day of rash onset. She had markedly elevated liver transaminases (ALT 520 U/L, normal < 65 U/L; AST 185 U/L, normal < 37 U/L), leukocytosis (WBC count 12,200/μL) and serum eosinophilia (18% eosinophils, eosinophil count 2,200/μL), but was afebrile. A clinical diagnosis of DRESS syndrome was made.
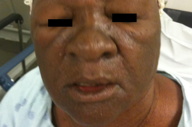 | 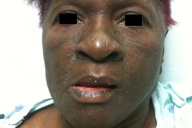 |
| Figure 1 | Figure 2 |
|---|
The patient was admitted and given intravenous methylprednisolone, intravenous diphenhydramine, and topical steroids. Her hypertension and hyperlipidemia medications were continued as an inpatient. After 3 days, her facial edema and erythema improved significantly (Figure 2) as did the rest of her exanthem. Her hospital stay was remarkable for intermittent episodes of shortness of breath, for which she received breathing treatments. Over several days, her liver transaminases slowly decreased, and she was discharged in good condition on a tapering prednisone course, topical steroids, oral diphenhydramine, and amlodipine. Initiation of an alternative anti-retroviral regimen was deferred.
Discussion
We report the first case in the dermatology literature of hypersensitivity syndrome related to raltegravir. Whereas the patient was started on ritonavir and darunavir at the same time, these are unlikely culprits because both are protease inhibitors, a relatively older and well-established class of anti-retroviral medications first introduced in 1995. Although associated with several dermatologic conditions including lipodystrophy and pyogenic granuloma-like lesions, protease inhibitors have not been reported in association with DRESS syndrome [7, 8]. Raltegravir is the first drug in a much newer class of medicines – integrase inhibitors – that has not been studied as extensively, and which has been previously shown to cause DRESS syndrome [6]. Our case suggests that clinicians should be aware of this potential adverse event when prescribing raltegravir.
References
1. Eshki M, Allanore L, Musette P, Milpied B, Grange A, Guillaume JC et al. Twelve-year analysis of severe cases of drug reaction with eosinophilia and systemic symptoms: a cause of unpredictable multiorgan failure. Arch Dermatol 2009;145:67-72. [PubMed]2. Peyriere H, Dereure O, Breton H, Demoly P, Cociglio M, Blayac JP et al. Variability in the clinical pattern of cutaneous side-effects of drugs with systemic symptoms: does a DRESS syndrome really exist? Br J Dermatol 2006;155:422-8. [PubMed]
3. Hetherington S, McGuirk S, Powell G, Cutrell A, Naderer O, Spreen B et al. Hypersensitivity reactions during therapy with the nucleoside reverse transcriptase inhibitor abacavir. Clin Ther 2001;23:1603-14. [PubMed]
4. Bourezane Y, Salard D, Hoen B, Vandel S, Drobacheff C , Laurent R. DRESS (drug rash with eosinophilia and systemic symptoms) syndrome associated with nevirapine therapy. Clin Infect Dis 1998;27:1321-2. [PubMed]
5. Angel-Moreno-Maroto A, Suárez-Castellano L, Hernández-Cabrera M, Pérez-Arellano JL. Severe efavirenz-induced hypersensitivity syndrome (not-DRESS) with acute renal failure. J Infect 2006;52:e39-e40. [PubMed]
6. Perry MEO, Almaani N, Desai N, Fox J, Larbalestier N , Chilton D. Raltegravir-induced drug reaction with eosinophilia and systemic symptoms (DRESS) syndrome: implications for clinical practice and patient safety. J Int AIDS Soc 2010;13 (Suppl 4): P110.
7. Ward HA, Russo GG , Shrum J. Cutaneous manifestations of antiretroviral therapy. J Am Acad Dermatol 2002;46:284-93. [PubMed]
8. Calista D , Boschini A. Cutaneous side effects induced by indinavir. Eur J Dermatol 2000;10:292-6.
© 2011 Dermatology Online Journal

