Acute generalized exanthematous pustulosis induced by nimesulide
Published Web Location
https://doi.org/10.5070/D39jh591qqMain Content
Acute generalized exanthematous pustulosis induced by nimesulide
M Teixeira, E Silva, M Selores
Dermatology Online Journal 12 (6): 20
Department of Dermatology, Hospital Geral de Santo António, Rua D. Manuel II, Edifício Ex-CICAP, Porto, Portugal. martamotateixeira@mail.telepac.ptAbstract
Nimesulide is a new nonsteroidal anti-inflammatory (NSAID) drug with antipyretic and analgesic properties. Because of its favorable tolerability profile it appears to be a preferred alternative, especially in NSAID-sensitive patients. We report a case of acute generalized exanthematous pustulosis (AGEP) in a 50-year-old woman that was attributed to the ingestion of nimesulide. To the best of our knowledge there have been no previous reports of AGEP induced by the ingestion of nimesulide in the medical literature. Nimesulide should be added to the list of agents associated with this serious adverse drug reaction.
Introduction
In 1968 review of 104 cases of pustular psoriasis, Baker and Ryan detected a clinically distinct subgroup of patients who had a very acute pustular eruption, drug intake, and no history of psoriasis. This was termed exanthematic pustular psoriasis [1]. Beylot et al.[2] introduced the term pustuloses exanthemátiques aiguës généralisées in 1980. Its English counterpart, acute generalized exanthematous pustulosis (AGEP), is currently used to refer to the condition.
Nimesulide is a new nonsteroidal anti-inflammatory (NSAID) drug with antipyretic and analgesic properties. Because of its favorable tolerability profile it appears to be an preferred alternative, especially in NSAID-sensitive patients [3]. Similar to other NSAIDs the most common reported adverse effects are epigastric pain, vomiting, heartburn, diarrhea, dizziness, somnolence, and headache [4]. Pruritus and rash are the most common cutaneous side effects associated with nimesulide [3, 4]. Purpura[5] and fixed drug eruptions [6, 7, 8] also are described but rare.
Clinical synopsis
 |
| Figure 2 |
|---|
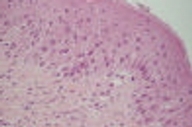
| Figure 2. Detail of histopathological examination of an early lesion revealed exocytosis of some neutrophils with spongiform epidermis. (H&E, x40) |
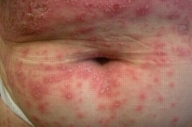
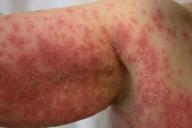
A 50-year-old woman was observed for an erythemato-pustular eruption that began 10 days after the intake of nimesulide for a sore throat. The eruption was associated with pruritus, severe malaise and hyperthermia (39°C). Physical examination revealed a diffuse symmetric erythema with numerous nonfollicular 1-2 mm pustules on the neck, limbs and trunk, more prominent and confluent in the axillae and submammary folds (Figs. 1A and 1B). The scalp, palms, soles and mucous membranes were spared. Blood tests showed leukocytosis (16.70×103/µl; NR=4.00-11.00×103/µl) with elevated neutrophil count (12.5×103/µl; NR=2.00-7.50×103/µl), elevated erythrocyte sedimentation rate (34mm/h; NR=0-19mm/h) and protein C reactive (8.36mg/dl; NR<0.5mg/dl). There was no hepatic cytolysis or renal failure. Viral serology revealed positive HBsAg, Anti-HBc and Anti-HBe and negative Anti-HBs, HBeAg and DeltaAg. Further studies confirmed the presence of chronic hepatitis B with no clinic, biologic or virulogical activity. She had no personal or family history of psoriasis or other diseases and she was on no other medications. Skin biopsy from an early lesion revealed exocytosis of some neutrophils with spongiform epidermis and a mixed interstitial and perivascular dermal inflammatory infiltrate composed of neutrophils, lymphocytes, and plasmocytes (Fig. 2). Direct immunofluorescence was negative as well as specific stains and cultures for bacteria and fungi. Based on the clinical history, laboratory, and histology findings the diagnosis of AGEP was established. Withdrawal of the presumed offending drug and administration oral hydroxyzine, topical betamethasone dipropionate cream, and emollients resulted in rapid clearing of the eruption with desquamation. One month after full recovery the patient was patch tested with the Portuguese Contact Dermatitis Group standard series and nimesulide (as is and 5 % in petrolatum). All tests were negative. There has been no recurrence of the skin lesions in a 2-year followup period.
Discussion
Acute generalized exanthematous pustulosis is characterized by an edematous erythema, mostly beginning on the intertriginous folds, that occurs very suddenly and on which a sterile pustular eruption develops. Patients often describe a burning or itching sensation. Skin symptoms are almost always accompanied by high fever (39-40°C) and leukocytosis (mostly neutrophilic) [9, 10, 11]. There are case reports that describe lymphadenopathy, slight reduction of creatinin clearance (<60ml/min in approximately 30 % of cases), and mild elevation of aminotranferases [12]. Two groups of patients have been identified with respect to the timing of onset after administration of the drug. In one group symptoms arise after 1-3 weeks (which is probably related to the primary sensitization); in the other group the delay is as short as a few hours to 3 days. According to the current data, males and females seem to be equally affected and AGEP can occur at any age. Interestingly, Bernard et al. demonstrated a higher frequency of HLA B51, DR11 e DQ3 in a group of 12 patients with AGEP when compared with the normal population. However the authors could not demonstrate any relation between the type of medication responsible for the adverse drug reaction and the HLA phenotype [13].
The histopathology of AGEP shows spongiform, subcorneal, or intraepidermal pustules, often marked edema of the papillary dermis, perivascular infiltrates of neutrophils, and exocytosis of some eosinophils. Acanthosis and papillomatosis resembling psoriasis are usually absent. Some keratinocyte cell necroses or vasculitis may be present [11, 14].
The diagnostic criteria initially proposed by Rojeau et al. [9] were recently revised by the EuroSCAR study group [10], and a validation score was elaborated based on morphologic and histologic criteria and disease course. The score gave 10 points to our patient and therefore AGEP was considered to be a definitive diagnosis. Drugs trigger this relative rare phenomenon in more than 90 percent of cases; antibiotics, especially β-lactams, are the most frequently implicated drugs, although a wide range of offending drugs is reported [9, 10, 11, 15]. Interestingly icodextrin [16] (peritoneal dialysis solution), iohexol [17] and iopamidol [18] (iodated contrast products), and radioactive thallium [19] (contrast product) have been recently added to the list of agents of AGEP. Only a minority of cases of acute viral infections, mainly enteroviruses [9, 20, 21] and also parvovirus B19 [22], are suspected of inducing AGEP. Other rarely reported etiologic agents are mercury [23, 24], pneumococcal vaccine [25], ingestion of lacquer chicken [26], and ultraviolet light exposure [27]. The finding of a chronic HBV infection did not seem to play a role in the induction of AGEP in our patient, because the clinical picture subsided without any specific treatment for this condition.
Although a precise pathophysiologic mechanism has not yet been identified for AGEP, several theories have been proposed. Britschini et al. [28] suggest the involvement of T cells, which is evidenced by positive patch tests and lymphocyte transformation tests. The cell-bound drug elicits a drug-specific CD4 and CD8 immune reaction producing interleukin 8 and interleukin 5. Besides their cytotoxic effect on basal cells these cytokines also provoke aggregation of neutrophils and eosinophils. Based on the positive patch test results, Moreau et al. [29] proposed that AGEP is a delayed type of hypersensivity reaction. Another possible mechanism is the production of antigen-antibody complexes induced by an infection or drug that activate the complement system, which in turn leads to neutrophil chemotaxis [11].
Theoretically the assessment of the culpability of a certain drug as the cause of AGEP can be obtained by rechallenging the patient with the suspected drug. However, oral rechallenge is not ethical because even at a low dose it may provoke a generalized eruption that can be even more serious then the initial clinical picture [30]. Patch testing has been a variably useful tool in identifying the etiologic agent in case of AGEP. The rationale for using the patch test in AGEP is founded mainly on its supposed pathogenesis, which, as mentioned above, includes T-cell involvement and a delayed-type hypersensivity reaction. Nevertheless, only 50 percent of AGEP cases demonstrate positive patch tests results, reproducing the original eruption clinically and histologically usually only at the patch test site [31]. Negative tests do not allow a final conclusion. Although the patch test reaction in most AGEP cases is limited to the test site, there are a few reports of reaction spreading beyond this site, most of them attributed to diltiazem hydrochloride [32]. Despite the value of patch test in the diagnosis of AGEP and the determination of the etiology, this method has drawbacks. There is no standardization for the tests whose specificity and sensitivity are yet to be determined. It is well known that sometimes, including in AGEP cases, a patch test can fail to provoke a reaction in the person who is sensitive to the substance tested. There are several reasons for these negative results, especially when the test is performed with specially prepared materials rather than standard test patches. Among the more common reasons are the different mechanisms of action of the drugs being tested, excessively low drug concentration, insufficient amount of applied drug, inappropriate vehicle, degradation of the substance, the culprit source of the adverse drug reaction was not the native drug itself but one of its metabolites, and UV degradation of the patch test site [29, 30, 31, 32, 33, 34]. In vitro tests, namely macrophage migration inhibitory factor and the mast cell degranulation tests, have confirmed the etiologic role of the suspected drugs in a group of six AGEP patients [35]. These tests confirm the immunological mechanism of AGEP and appear as a valuable promising diagnostic aid. Another advantage over the in vivo tests (patch tests and readministration) is the absence of risk of severe allergic reaction in the patients. However studies including larger groups of patients are needed for definitive conclusions.
Although AGEP is a potentially severe disease with up to 5 percent mortality, it generally has a good overall prognosis. Nevertheless high fever or superinfection of skin lesions can sometimes lead to life-threatening situations in elderly patients or those with poor general condition. After withdrawal of the presumed offending drug, pustules resolve spontaneously in less then 15 days, generally with a characteristic post-pustular pin-point extensive desquamation [36]. Symptomatic systemic medications such as anti-pyretics and anti-histamines can be prescribed, if not suspected as causative drugs for the disease. The diagnosis of AGEP should be made early enough so that unnecessary investigations and the administration of aggressive therapies for other suspected pustular eruptions can be avoided.
Because it is essential to discontinue the causative drug as soon as possible if a pustular eruption occurs, physicians must be informed of the risk, which should be added to adverse effects and warning sections of the summary of product characteristics of the drug concerned. Therefore notification of side effects by physicians to the pharmacovigilance centers is crucial [37].
Although the patch tests were negative in our patient, the clinical and histological findings suggest a diagnosis of AGEP induced by nimesulide. To the best of our knowledge there are no previous reports of AGEP induced by the ingestion of nimesulide in the medical literature. We believe that nimesulide should be added to the list of causes of this serious adverse drug reaction.
References
1. Baker H, Ryan TJ. Generalized pustular psoriasis. A clinical and epidemiological study of 104 cases. Br J Dermatol 1968; 80: 771-793.2. Beylot C, Bioulac P, Doutre MS. Pustuloses exanthématiques aiguës généralisées, à propos de 4 cas. Ann Dermatol Venereol 1980; 107: 37-48.
3. Bavbek S, Celik G, Ediger D et al. The use of nimesulide in patients with acetylsalicylic acid and nonsteroidal anti-inflammatory drug intolerance. J Asthma 1999; 36: 657-663.
4. Davis R, Brogden RN. Nimesulide. An update of its pharmacodynamic and pharmacokinetic properties, and therapeutic efficacy. Drugs 1994; 48: 431-454.
5. Kanwar AJ, Kaur S, Thami GP. Nimesulide-induced purpura. Dermatology 2000; 201: 376.
6. Valsecchi R, Reseghetti A, Cainelli T. Bullous and erosive stomatitis induced by nimesulide. Dermatology 1992; 185: 74-75.
7. Cutaneous reactions to analgesic-antipyretics and nonsteroidal anti- inflammatory drugs. Analysis of reports to the spontaneous reporting system of the Gruppo Italiano Studi Epidemiologici in Dermatologia. Dermatology 1993; 186: 164-1699.
8. Cordeiro MR, Goncalo M, Fernandes B et al. Positive lesional patch tests in fixed drug eruptions from nimesulide. Contact Dermatitis 2000; 43: 307.
9. Roujeau JC, Bioulac-Sage P, Bourseau C et al. Acute generalized exanthematous pustulosis. Analysis of 63 cases. Arch Dermatol 1991; 127: 1333-1338.
10. Sidoroff A, Halevy S, Bavinck JN et al. Acute generalized exanthematous pustulosis (AGEP): a clinical reaction pattern. J Cutan Pathol 2001; 28: 113-119.
11. Beylot C, Doutre MS, Beylot-Barry M. Acute generalized exanthematous pustulosis. Semin Cutan Med Surg 1996; 15: 244-249.
12. Eeckhout I, Noens L, Ongenae K et al. Acute generalized exanthematic pustulosis: a case with a lymphoma-like presentation. Dermatology 1997; 194: 408-410.
13. Bernard P, Lizeaux-Parneix V, Miossec V et al. HLA et predisposition génétique dans les pustulosis exanthématiques (PEAG) et les exánthemes maculo-papuleux (EMP). Ann Dermatol Venereol 1995; 122: S38.
14. Burrows NP, Russell Jones RR. Pustular drug eruptions: a histopathological spectrum. Histopathology 1993; 22: 569-573.
15. Saissi EH, Beau-Salinas F, Jonville-Bera AP et al; Centres Regionaux de Pharmacovigilance. Drugs associated with acute generalized exanthematic pustulosis. Ann Dermatol Venereol 2003; 130: 612-618.
16. Al-Hoqail IA, Crawford RI. Acute generalized exanthematous pustulosis induced by icodextrin. Br J Dermatol 2001; 145: 1026-1027.
17. Atasoy M, Erdem T, Sari RA. A case of acute generalized exanthematous pustulosis (AGEP) possibly induced by iohexol. J Dermatol 2003; 30: 723-726.
18. Belgodere X, Wolkenstein P, Pastor MJ. Pustulose exanthématique aiguës généralisée induite par iopamidol. Ann Dermatol Venereol 2004; 131: 831-832.
19. Aziz Jalali MH, Mirzazadeh Javaheri S, Salehian P Acute generalized exanthematous pustulosis due to thallium. J Eur Acad Dermatol Venereol 2004; 18: 321-323.
20. Rouchouse B, Bonnefoy M, Pallot B et al. Acute generalized exanthematous pustular dermatitis and viral infection. Dermatologica 1986; 173: 180-184.
21. Feio AB, Apetato M, Costa MM et al. Acute generalized exanthematous pustulosis due to Coxsackie B4 virus. Acta Med Port 1997; 10: 487-491.
22. Perceau G, Derancourt C, Salmon-Ehr V et al. Acute generalized exanthematous pustulosis in hypercalcemia. Ann Dermatol Venereol 2000; 127: 1090-1093.
23. Bolzinger T, Ducombs G, Labreze C et al. Acute generalized exanthematous pustulosis in a child and epicutaneous tests with mercurials. Ann Dermatol Venereol 1993; 120: 223-225.
24. Barrazza V, Meunier P, Escande JP. Acute contact dermatitis and exanthematous pustulosis due to mercury. Contact Dermatitis 1998; 38: 361.
25. Correia O, Nunes JP, Vaz-da-Silva MJ et al. Acute exanthematous pustular dermatitis after pneumococcal vaccine ?Letter?. Dermatology 1993; 187: 217.
26. Park YM, Park JG, Kang H et al. Acute generalized exanthematous pustulosis induced by ingestion of lacquer chicken. Br J Dermatol 2000; 143: 230-232.
27. Yip J, Sheehan-Dare R, Cotterill J. Toxic pustuloderma due to PUVA treatment Letter. Br J Dermatol 1991; 125: 401-402.
28. Britschgi M, Steiner UC, Schmid S et al. T-cell involvement in drug-induced acute generalized exanthematous pustulosis. J Clin Invest 2001; 107: 1433-1441.
29. Moreau A, Dompmartin A, Castel B et al. Drug-induced acute generalized exanthematous pustulosis with positive patch tests. Int J Dermatol 1995; 34: 263- 266.
30. Tsuda S, Kato K, Karashima T et al. Toxic pustuloderma induced by ofloxacin. Acta Derm Venereol 1993; 73: 382-384.
31. Wolkenstein P, Chosidow O, Flechet ML et al. Patch testing in severe cutaneous adverse drug reactions, including Stevens-Johnson syndrome and toxic epidermal necrolysis. Contact Dermatitis 1996; 35: 234-236.
32. Mashiah J, Brenner S. A systemic reaction to patch testing for the evaluation of acute generalized exanthematous pustulosis. Arch Dermatol 2003; 139: 1181- 1183.
33. Begaud B, Evreux JC, Jouglard J et al. Imputation of the unexpected or toxic effects of drugs. Actualization of the method used in France. Thérapie 1985; 40: 111-118.
34. Watsky KL. Acute generalized exanthematous pustulosis induced by metronidazole: the role of patch testing. Arch Dermatol 1999; 135: 93-94.
35. Lazarov A, Livni E, Halevy S. Generalized pustular drug eruptions: confirmation by in vitro tests. J Eur Acad Dermatol Venereol 1998; 10: 36-41.
36. Rojeau, JC. Clinical heterogeneity of drug hypersensitivity. Toxicology 2005; 209: 123-129.
37. Thiessard F, Roux E, Miremont-Salame G et al. Trends in spontaneous adverse drug reaction reports to the French pharmacovigilance system (1986-2001). Drug Saf 2005; 28: 731-740.
© 2006 Dermatology Online Journal