Melanoma-associated leukoderma
Published Web Location
https://doi.org/10.5070/D39hh536dxMain Content
Melanoma-associated leukoderma
Elizabeth K Hale MD and Jody W Konstadt MD
Dermatology Online Journal 9(4): 20
From the Ronald O. Perelman Department of Dermatology, New York University
Abstract
A case is presented of a man with a history of melanoma treated with sentinel lymphadenectomy and interferon therapy, who subsequently developed diffuse hypopigmented patches thought to be consistent with a diagnosis of melanoma-associated leukoderma. Clinically, melanoma-associated leukoderma is a diffuse macular hypomelanosis or depigmentation, which often develops at sites distant to the location of the primary melanoma. The leukoderma may be hypomelanotic and mottled or depigmented and milk-white. Spontaneous repigmentation may occur. A recent study has demonstrated that T cells involved in the destruction of neoplastic melanocytes are identical clones of those that accumulate in melanoma-associated leukoderma.
Clinical summary
History.—A 51-year-old man presented with a 1-year history of hypopigmentation involving the face, shoulders, torso, and lower extremities. The patient was referred to the Charles C. Harris Skin and Cancer Pavilion for evaluation of white patches, which developed after the diagnosis and treatment of a 1.8-mm melanoma. The initial diagnosis of melanoma on his mid-back was made in April 2000. The treatment was excision and sentinel lymph node dissection and subsequently intravenous and subcutaneous interferon therapy for 1 year. Approximately 1 year ago, he first noticed white patches on his shoulders, torso, legs and face. A skin biopsy was not performed. Since that time, he has noticed that some of the white areas have repigmented spontaneously.
Past medical history includes hypertension and hypercholesterolemia. Enalapril, atenolol, and pravastatin had been administered for several years prior to the occurrence of cutaneous depigmentation.
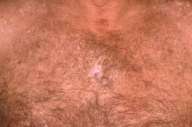
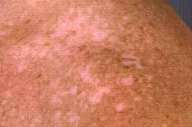
Physical examination.—Depigmented patches were noted diffusely, most prominently on the face, shoulders, chest, and lower extremities. A scar on the back at the site of the melanoma excision failed to show evidence of local recurrence or satellitosis. There was no lymphadenopathy or hepatosplenomegaly.
|
|
| Figure 1 | Figure 2 |
|---|
Laboratory data.—Wood's lamp examination enhanced the numerous, discrete, white macules and patches.
Histopathology.—None
Diagnosis.— Melanoma-associated leukoderma.
Comment
There are several types of hypomelanosis that may occur in patients with melanoma. Loss of pigmentation may involve the primary lesion itself and take the form of an eccentrically placed hypopigmented macule, analogous to a halo nevus, or it may occur in distant locations, which results in a process that is known as melanoma-associated leukoderma. The leukoderma observed in patients with melanoma may be the result of an immunologic response against abnormal melanocytes [1]. Some evidence suggests that it may confer a more favorable prognosis for the patient. One retrospective study of Mexican patients with melanoma-associated leukoderma found that affected patients have a significantly higher than expected survival, with respect to the thickness of the associated primary melanoma [2].
Clinically, melanoma-associated leukoderma is a diffuse, macular hypomelanosis or depigmentation. It often develops at sites distant from the primary melanoma and is frequently first observed on the trunk, with subsequent spread to the extremities. Spontaneous repigmentation may occur.
Immunologic destruction of tumors is mediated primarily by lymphocytes. Activated T cells exert this effect by recognizing peptides derived from endogenous proteins [1]. For melanoma, these proteins may be either antigens encoded by tumor-specific or mutated genes or melanocytic differentiation antigens, which are normal proteins restricted to the melanocyte lineage. Some examples of melanocytic differentiation antigens are tyrosinase, Melan-A/MART-1, and gp 100; T cells specific to these antigens are found in the majority of melanoma patients. The inability to accurately distinguish self from tumor antigens is the basis of clinical trials using peptides to vaccinate melanoma patients to induce therapeutic antitumor immunity [3]. The association between vitiligo-like leukoderma, resulting from destruction of normal melanocytes, and melanoma regression, resulting from destruction of cancer cells, suggests that targets of immunotherapy involved in melanoma regression comprise normal differentiation antigens. A study utilizing reverse transcription-polymerase chain reaction and denaturing gradient gel electrophoresis has demonstrated that T cells involved in the destruction of neoplastic melanocytes are identical clones of those that accumulate in melanoma-associated leukoderma [4].
References
1. Kirkin AF, et al. The immunologic properties of melanoma-associated antigens recognized by cytotoxic T lymphocytes. Exp Clin Immunogent 1998;15:19.2. Rodrigues-Cuevas S, et al. Prognostic significance of cutaneous depigmentation in Mexican patients with malignant melanoma. Arch Med Res 1998;29:155.
3. Rosenberg SA, et al. Immunologic and therapeutic evaluation of a synthetic peptide vaccine for the treatment of patients with metastatic melanoma. Nature Med 1998;4:321.
4. Becker JC, et al. Accumulation of identical T cells in melanoma and vitiligo-associated leukoderma. J Invest Dermatol 1999;113:1033.
© 2003 Dermatology Online Journal

