Treatment of mycosis fungoides using a 308-nm excimer laser: Two case studies
Published Web Location
https://doi.org/10.5070/D398s4364wMain Content
Treatment of mycosis fungoides using a 308-nm excimer laser: Two case studies
John L Meisenheimer MD
Dermatology Online Journal 12 (7): 11
South Orlando Dermatology, Orlando, Florida. luckyj@msn.com
Abstract
Early-stage mycosis fungoides (MF) is most commonly treated with skin-directed therapies such as topical steroids, phototherapy (broadband or narrowband UVB), photochemotherapy (psoralen plus UVA), topical nitrogen mustard, and total skin electron-beam irradiation. Recently, several small studies have demonstrated the efficacy of the 308-nm excimer laser in the treatment of early-stage MF. This xenon-chloride laser, which emits monochromatic excimer light at the 308-nm wavelength, has been approved by the Food and Drug Administration to treat psoriasis since 1997 and to treat vitiligo since 2001. We report two patients in which patch-stage MF was treated with a 308-nm excimer laser. Our findings confirm previous observations that the 308-nm excimer laser is a safe, effective, and well-tolerated therapy for early stage MF.
Introduction
Mycosis fungoides (MF) is the most common form of cutaneous T-cell lymphoma (CTCL). In its early stages (IA and IB), MF is most commonly treated with skin-directed therapies, which include topical steroids, phototherapy (broadband or narrowband UVB), photochemotherapy (psoralen plus UVA, aka PUVA), topical nitrogen mustard, and total skin electron-beam irradiation [1].
The efficacy of phototherapy in treating the patch stage of MF has been well documented. Good results with broadband-UVB phototherapy (280-320-nm wavelength) and narrowband UVB phototherapy (311 nm) have been reported [2, 3, 4, 5, 6, 7, 8, 9, 10].
Recently, several small studies demonstrated the efficacy of 308-nm excimer lasers in the treatment of early-stage MF [11, 12, 13, 14]. In our study we used the XTRAC model AL7000 laser (PhotoMedex, Montgomeryville, Pennsylvania), which emits monochromatic excimer light at the 308-nm wavelength and provides a spot size of 4cm2 with a power density of 462 mW/cm2. The XTRAC excimer laser has been approved by the Food and Drug Administration to treat psoriasis since 2000 and to treat vitiligo since 2001 [15, 16].
We report two patients with patch-stage mycosis fungoides treated with a 308-nm excimer laser (XTRAC Excimer Laser; PhotoMedex, Montgomeryville, Pennsylvania).
Clinical synopsis
Patient 1
A 76-year-old woman with a several-month history of a nonspecific erythematous patch on the right thigh presented for evaluation. A potassium hydroxide test was negative. A biopsy was taken to rule out MF versus chronic contact dermatitis. She was started on diflorasone diacetate ointment 0.05 percent to be applied to the affected area until biopsy results were received.
Histology showed an atypical lymphoid infiltrate at the dermal-epidermal interface, which was most compatible with patch-stage MF. The patient was referred to an oncologist to rule out internal involvement; all scans were clear.
Because this was a solitary patch (diameter 8 cm), local treatment was recommended. The patient elected treatment with the 308-nm excimer laser.
Before treatment, she was phototested to determine the minimal erythema dose (MED) of 308-nm UVB by exposing an uninvolved area of skin to a dose range between 100 and 350 mJ/cm2.
The first treatment session started at the MED (~100 mJ/cm2), and increased gradually to 300 mJ/cm2 per session. Sessions were twice a week, separated by 2-5 days. The area treated varied in size from 80 cm2 to 16 cm2 (mean 59 cm2) over the treatment sessions. The total UVB 308-nm irradiation dose was 3.25 J/cm2 (Table 1). After 14 sessions, there was complete clinical clearing, and treatment was stopped. Post-treatment histology showed no evidence of residual mycosis fungoides.
The patient returned 6 months later for follow-up. The treated patch was still clinically clear, and all hyperpigmentation from the laser treatment was also gone. She had no new lesions suggestive of MF and showed no signs of lymphadenopathy. She will return at 6-month intervals for follow-up.
Patient 2
A 56-year-old woman with a 3-year history of limited patch-stage MF presented at followup with two new patches. She had been responsive to PUVA therapy in the past, confirmed by histology. The patient had previously been examined by an oncologist; all scans were clear. At the time of this follow-up, the patient had been clinically clear of MF for 15 months.
|
|
| Figure 1 | Figure 2 |
|---|---|
|
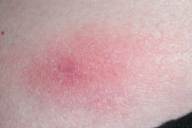
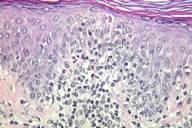
Figure 1. Right posterior waist patch before excimer laser treatment Figure 2. Right posterior waist patch—histology before excimer laser treatment |
|
The two new patches, right posterior waist (Figs. 1 and 2) and right torso, were clinically consistent with MF. Histology for both patches showed an abnormal lymphocytic infiltrate consistent with residual MF.
Although the patient had responded to past courses of PUVA therapy, she had experienced nausea and vomiting with the treatments. She had also been unresponsive to topical bexarotene in the past. She requested other treatment possibilities.
The option of UVB treatments using the 308-nm excimer laser was discussed with the patient. She elected laser treatment.
Before treatment, she was phototested to determine the minimal erythema dose (MED). Treatment started at 150 mJ/cm2, and then gradually increased to 1000 mJ/cm2. Sessions were twice a week, separated by 3-5 days. The total area treated varied in size from 8 cm2 to 12 cm2 over the treatment sessions. The total UVB 308-nm irradiation dose was 9.9 J/cm2 (Table 1). After 16 sessions, there was clinical improvement in both areas, and treatment was stopped. However, a new patch appeared on the right thigh that was clinically consistent with MF.
Biopsies were taken of the new patch and the two patches that had been laser-treated. The treated torso site showed clearing of abnormal lymphocytes and required no further laser sessions. The treated patch on the right posterior waist still showed abnormal lymphocytes. The new right thigh patch was consistent with MF.
Treatment on the new thigh patch started at 150 mJ/cm2 and then ranged between 300 and 450 mJ/cm2. Treatment on the right posterior waist patch, which had previously received 16 excimer laser treatments, continued at 750 mJ/cm2, and was reduced to 150-200 mJ/cm2 for the final 4 sessions because of sustained erythema at the site. The total UVB 308-nm irradiation dose was 7.5 J/cm2 (Table 1). After 14 sessions, both sites (thigh and waist) appeared clinically clear.
Post-treatment histology showed that the treated thigh site was negative for persistent MF. The posterior waist site, after a total of 30 treatment sessions, continued to show signs of minimally persistent patch-stage MF (Table 1).
The waist site received 8 more laser sessions, administered twice a week. The total UVB 308-nm irradiation dose was 3.0 J/cm2. Post-treatment histology showed no evidence of residual MF after 38 treatment sessions (Table 1).
At the next 6-month follow-up visit, the previously treated sites still showed clinical clearing, but two new patches had developed on the right thigh. Histology revealed atypical lymphocytes consistent with MF at both sites.
These two patches received 14 laser sessions, administered twice a week. The total area treated varied in size from 40 cm2 to 52 cm2 over the treatment sessions. The total UVB 308-nm irradiation dose was 6.7 J/cm2 (Table 1).
Post-treatment histology showed no evidence of residual mycosis fungoides in the two laser-treated areas. Biopsies were also taken at two new unrelated sites, a patch on the lower right back and a lesion on the left shoulder. The two new sites were negative for MF.
At the next 6-month follow-up visit, the patient presented with four new patches and a possible recurrence at the posterior waist site, which had previously received 38 laser treatments followed by a biopsy that was MF negative. The five sites were biopsied. The waist site showed a recurrence of MF. Of the four new sites, one was negative for MF. The other three (right buttock, left posterior thigh, right upper thigh) were consistent with persistent MF.
|
|
| Figure 3 | Figure 4 |
|---|---|
|
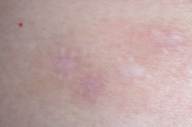
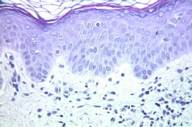
Figure 3. Right posterior waist patch after cumulative dose of 25 J/cm2 Figure 4. Right posterior waist patch—histology after cumulative dose of 25 J/cm2 |
|
Again, the patient elected excimer laser treatment. These four patches received 17 laser sessions, administered twice a week. The total area treated averaged 32 cm2. The total UVB 308-nm irradiation dose was 4.6 J/cm2 (Table 1). Post-treatment histology showed no evidence of MF at the waist site (after a total of 55 treatment sessions) (Figs. 3 and 4); the other three sites were also negative for MF.
At the time of the post-treatment histology, a new patch on the right lateral abdomen was observed and sampled. This new site showed persistent MF. At the time of this writing, the new patch on the abdomen has received 3 treatments at 150 mJ/cm2 per session. Sessions will continue until clinical clearing.
Discussion
Over the past few years, several studies regarding the use of 308-nm excimer lasers in the treatment of early stage MF (stages IA and IB) have been published. Although treatment protocols varied, principally in the frequency of laser sessions that patients received per week, the mean cumulative dose required for clearance was similar, ranging from 7.0 J/cm2 to 9.4 J/cm2 [11, 12, 13, 14]. In our small study, the mean cumulative dose for clearance was 5.8 J/cm2, although Patient 2 had a patch (right posterior waist) that ultimately required a cumulative dose of 25 J/cm2 for clearance.
In contrast, the mean cumulative dose of narrowband (311-nm) UVB required for clearance, as reported in several studies, was found to be 17.2 - 96.7 J/cm2, depending on the study [4, 5, 6, 7, 10]. This suggests that the cumulative dose of 308-nm UVB required to clear MF patches may be significantly less than for narrowband UVB. And, unlike most narrowband UVB systems, which expose large areas of skin to UVB rays, the 308-nm excimer laser handpiece is able to precisely target MF patches while sparing normal skin from unnecessary radiation exposure.
Arguably, PUVA therapy may have been a more cost-effective option for Patient 2. But because she had experienced nausea and vomiting in the past with PUVA, she elected to try a treatment with fewer immediate (and possibly long-term) side effects. Long-term high-dose PUVA therapy is associated with an increased risk of skin malignancies such as squamous or basal cell carcinoma [17, 18].
Like PUVA and all other treatments for MF, 308-nm UVB is probably not a cure but another option for managing MF. Our findings confirm previous observations that the 308-nm excimer laser is a safe, effective, and well-tolerated therapy for early stage MF.
More follow-up is needed to chart the course of relapses following excimer laser treatment. Larger controlled clinical trials are also needed to further confirm the efficacy of the 308-nm excimer laser as first-line treatment for patch-stage MF and to establish optimum protocols.
References
1. Apisarnthanarax N, Talpur R, Duvic M. Treatment of cutaneous T cell lymphoma: current status and future directions. Am J Clin Dermatol. 2002;3(3):193-215. PubMed.2. Ramsay DL, Lish KM, Yalowitz CB, Soter NA. Ultraviolet-B phototherapy for early stage cutaneous T-cell lymphoma. Arch Dermatol. 1992 Jul;128(7):931-33. PubMed.
3. Resnik KS, Vonderheid EC. Home UV phototherapy of early mycosis fungoides: long term follow-up observations in thirty one patients. J Am Acad Dermatol. 1993 Jul(1);29:73-7. PubMed.
4. Hofer A, Cerroni L, Kerl H, Wolf P. Narrowband (311 nm) UVB therapy for small plaque parapsoriasis and early-stage mycosis fungoides. Arch Dermatol. 1999 Nov;135(11):1377-80. PubMed.
5. Clark C, Dawe RS, Evans AT et al. Narrowband TL 01 phototherapy for patch-stage mycosis fungoides. Arch Dermatol. 2000 Jun;136(6):748-752. PubMed.
6. Gathers RC, Scherschun L, Malick F, Fivenson DP, Lim HW. Narrowband UVB phototherapy for early-stage mycosis fungoides. J Am Acad Dermatol. 2002 Aug;47(2):191-7. PubMed.
7. Diederen PV, van Weelden H, Sanders CJG, Toonstra J, van Vloten WA. Narrowband UVB and psoralen-UVA in the treatment of early-stage mycosis fungoides: a retrospective study. J Am Acad Dermatol. 2003 Feb;48(2):215-219. PubMed.
8. El-Mofty M, El-Darouty M, Salonas M, et al. Narrow band UVB (311 nm), psoralen UVB (311 nm) and PUVA therapy in the treatment of early-stage mycosis fungoides: a right-left comparative study. Photodermatol Photoimmunol Photomed. 2005 Dec;21(6):281-6. PubMed.
9. Boztepe G, Sahin S, Ayhan M, Erkin G, Kilemen F. Narrowband ultraviolet B phototherapy to clear and maintain clearance in patients with mycosis fungoides. J Am Acad Dermatol. 2005 Aug;53(2):242-6. PubMed.
10. Ghodsi SZ, Hallaji Z, Balighi K, Safar F, Chams-Davatchi C. Narrow-band UVB in the treatment of early stage mycosis fungoides: report of 16 patients. Clin Exp Dermatol. 2005 Jul;30(4):376-8. PubMed.
11. Nistico S, Costanzo A, Saraceno R, Chimenti S. Efficacy of monochromatic excimer laser radiation (308 nm) in the treatment of early stage mycosis fungoides. Br J Dermatol. 2004 Oct;151(4):877-9. PubMed.
12. Passeron T, Zakaria W, Ostovari N, Perrin C, Larrouy JC, Lacour JP, Ortonne JP. Efficacy of the 308-nm excimer laser in the treatment of mycosis fungoides. Arch Dermatol. 2004 Oct;140(10):1291-3. PubMed.
13. Mori M, Campolmi P, Mavilia L, Rossi R, Cappugi P, Pimpinelli N. Monochromatic excimer light (308 nm) in patch-stage IA mycosis fungoides. J Am Acad Dermatol. 2004 Jun;50(6):943-5. PubMed.
14. Upjohn E, Lane P, Foley p, Sinclair R, Magee J. Histological clearance of patch stage mycosis fungoides with the 308 nm excimer laser. 63rd Annual Meeting of the American Academy of Dermatology, New Orleans, LA, 2004. Poster #15.
15. U.S. Food and Drug Administration. Center for Devices and Radiological Health. 510K Approval #K992914. Issued January 27, 2000.
16. U.S. Food and Drug Administration. Center for Devices and Radiological Health. 510K Approval #K003705. Issued March 1, 2001.
17. Stern RS, Bolshakov S, Nataraj AJ, et al. p53 mutation in nonmelanoma skin cancers occurring in psoralen ultraviolet A-treated patients: evidence for heterogeneity and field cancerization. J Invest Dermatol. 2002 Aug;119(2):522-6. PubMed.
18. Nijsten TE, Stern RS. The increased risk of skin cancer is persistent after discontinuation of psoralen + ultraviolet A: a cohort study. J Invest Dermatol. 2003 Aug; 121(2):252-8. PubMed.
© 2006 Dermatology Online Journal

