A case of erythema multiforme developing after levetiracetam therapy
Main Content
A case of erythema multiforme developing after levetiracetam therapy
Yavuz Yesilova MD, Enver Turan, Abdurahman Sonmez, Ilyas Ozardali
Dermatology Online Journal 19 (2): 12
Harran University School of Medicine, Department of Dermatology, Sanliurfa, TurkeyAbstract
Erythema multiforme is a skin disease, which occurs particularly in the acral region, and is characterized by target-like erythematous macules and papules. Infections play an important role in the etiology of erythema multiforme. Other causes include drugs, vaccination, and hematological malignancies. Half of all cases may not have an identifiable etiology. This article presents a male patient who developed erythema multiforme as a result of levetiracetam use for epilepsy.
Introduction
 |  |
| Figure 1 | Figure 2 |
|---|
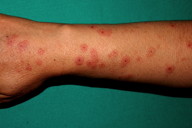
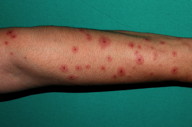
A 27-year-old female patient came to our clinic with a red, itchy eruption on the dorsal areas of both hands for five days. The patient had no systemic disease, but she had been receiving oral levetiracetam (1,000 mg/day) treatment for epilepsy. Fifteen days after initiation of the treatment she developed the skin complaints. She had been taking no medication for infection or systemic disease before starting the levetiracetam. Physical examination revealed target-shaped erythematous papules and plaques on the dorsum of both of her hands and extensor forearms (Figures 1 and 2). Her vital signs and systemic examination results were normal.
No pathological findings were observed of the cardiovascular, respiratory, gastrointestinal and neurological systems. White blood cell count was 9300/mm³, and laboratory tests showed eosinophil 0.5 percent, 32 percent polymorph, 32 percent lymphocyte, and 1.1 percent monocyte. Coagulation parameters were within normal limits. SGOT was 22U/l, SGPT 17U/L, urea 12mg/dl, creatinine 0.8 mg/dl, CRP 1.2 mg/dl, and sedimentation was 14 mm/hour. Serological tests showed negative results related to mycoplasma pneumonia, Epstein-Barr, and herpes simplex viruses. Blood cultures were negative. RF, ANA, and ANCA tests were negative.
 |
| Figure 3 |
|---|
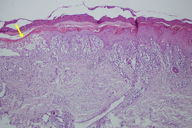
| Figure 3. The epidermis exhibits necrotic keratinocytes, general spongiosis, apoptosis, and lymphocyte exocytosis. |
Histological findings were typical of erythema multiforme and showed necrotic keratinocytes, spongiosis, and lymphocyte exostosis. Dense edema in the upper dermis and mononuclear inflammatory cell infiltration were also observed (Figure 3). Based on clinical and histopathological findings, the case was diagnosed as erythema multiforme (EM). The patient was monitored as a clinic inpatient and was medicated mainly with intravenous prednisolone at 1 mg/kg/day. Her antiepileptic treatment was stopped. After levetiracetam was discontinued, 400 mg carbamazepine treatment was initiated. The eruption abated and the patient was discharged after her oral prednisolone treatment had been decreased. The skin lesions of the patients did not relapse.
Infections, medicines, physical agents, x-ray treatment, pregnancy, and internal cancers are implicated in the etiopathogenesis of EM. The most common offending drugs include sulfonamides, non- steroidal anti-inflammatory drugs, antituberculous drugs, antibiotics, pyrazolones, phenylbutazone, oxyphenbutazone, phenazone and salicylates, and barbiturates and antiepileptic drugs (AEDs) [1]. AEDs that are used in epilepsy prophylaxis may cause cutaneous drug reactions varying from maculopapular eruption to SJS and TEN. Cutaneous drug reactions resulting from aromatic drugs such as phenobarbital, phenytoin, oxcarbazepine, carbamazepine, primidone, zonisamide, and lamotrigine are common, whereas cutaneous drug reactions attributed to second-generation antiepileptic drugs like gabapentin, tiagabine, topiramate, valproate and levetiracetam are rare [2].
Levetiracetam is a new AED with proven effectiveness in treating both partial and generalized seizures [2]. As far as we know, the literature includes just two studies that report cutaneous drug reactions following levetiracetam use. One report showed that, out of 1,890 patients, 2.8 percent had developed cutaneous side effects associated with the use of antiepileptic drugs. Maculopapular cutaneous side effects were observed in 0.6 percent of the patients who had used levetiracetam. But SJS-type cutaneous side effects were not observed in any of the patients who had been using levetiracetam [3]. However, Beswick et al did report reticular-style rashes following levetiracetam tb use in a 46-year-old female patient with a brain tumor [2]. In addition, Cotton et al and Patwardhan et al recommended the use of levetiracetam for patients who had developed cutaneous side effects like SJS and cutaneous hypersensitivity reactions following aromatic anticonvulsant drugs such as phenytoin and carbamazepine [4, 5]. To reduce the risk of allergic reactions, AED drugs are usually initiated in low doses, then the dose is gradually increased [2]. The initial dose of levetiracetam, in our case, was 500 mg/day and no skin reaction was observed during that period. The cutaneous side effects were observed after the dosage had been increased to 1,000 mg/day.
Levetiracetam is a new antiepileptic drug used in both partial and generalized seizure disorders. With the serious cutaneous and systemic side effects associated with anticonvulsants in the aromatic group, called the classical antiepileptics, new-generation antiepileptics are now regarded as alternative treatments for this disease. Although they are considered to be safe, in spite of the two cutaneous side effects reported for levetiracetam, EM was clearly observed in our case. Therefore, there is a need for greater awareness of both the systemic and the cutaneous side effects of levetiracetam.
References
1. Lamoreux MR, Sternbach MR, Hsu WT. Erythema multiforme. Am Fam Physician 2006 Dec 1;74(11):1883-8. [PubMed]2. Beswick TC, Cohen JB. Dose-related levetiracetam-induced reticulated drug eruption. J Drugs Dermatol 2010 Apr;9(4):409-10. [PubMed]
3. Arif H, Buchsbaum R, Weintraub D, Koyfman S, Salas-Humara C, Bazil CW, Resor SR, Hirsch LJ. Comparison and predictors of rash associated with 15 antiepileptic drugs. Neurology 2007 May 15;68(20):1701-9. [PubMed]
4. Cotton BA, Kao LS, Kozar R, Holcomb JB. Cost-utility analysis of levetiracetam and phenytoin for posttraumatic seizure prophylaxis. J Trauma 2011 Aug;71(2):375-9. [PubMed]
5. Patwardhan RV, Dellabadia J, Rashidi M, Grier L, Nanda A. Control of refractory status epilepticus precipitated by anticonvulsant withdrawal using left vagal nerve stimulation: a case report. Surg Neurol 2005 Aug;64(2):170-3. [PubMed]
© 2013 Dermatology Online Journal


