Porphyria cutanea tarda induced by tamoxifen
Published Web Location
https://doi.org/10.5070/D38xm7n81bMain Content
Porphyria cutanea tarda induced by tamoxifen
Maria J Cruz MD, Sergio Alves MD, Teresa Baudrier MD, Filomena Azevedo MD
Dermatology Online Journal 16 (9): 2
Hospital de São JoãoAbstract
Porphyria cutanea tarda (PCT) results from a decrease in the activity of uroporphyrinogen decarboxylase. In the sporadic form, the decrease in the activity is restricted to the liver and is generally related to alcohol, estrogens, iron overload, hepatitis C infection, and halogenated aromatic hydrocarbons. We describe the development of porphyria cutanea tarda in a 53-year-old woman one year after breast cancer surgery and the initiation of treatment with tamoxifen. No additional drugs were prescribed. After tamoxifen was discontinued, a gradual clinical and laboratorial improvement was noticed suggesting a causative role of the drug. There are many reports discussing tamoxifen side-effects, but there are only three case reports in the literature that describe tamoxifen as a probable trigger of porphyria cutanea tarda. In this report, the potential porphyrinogenicity of tamoxifen and clinical implications are the targets of our discussion.
Introduction
Porphyria cutanea tarda (PCT) is the most common form of porphyria and arises from a deficiency of uroporphyrinogen decarboxylase (UROD). In overt PCT, there is accumulation of uroporphyrinogen and other polycarboxylated intermediates in the liver that are oxidized to the correspondent porphyrins. These are then released from the liver and circulate in plasma to the skin and lead to phototoxic reactions on sun-exposed areas. Skin fragility, bullous lesions, and hypertrichosis are characteristic of the clinical phenotype of PCT [1]. Two variants, designated type I and type II, constitute virtually all cases of PTC in adults. The most common is type I (sporadic PCT), responsible for up to 80 percent of all cases, in which UROD deficiency is restricted to the liver. In type II (familial PCT), transmitted as an autosomal dominant trait, mutations in the UROD gene are present and enzyme activity is reduced in all tissues. Variable clinical expression is seen in familial-PCT and in the absence of aggravating factors the mutation is rarely sufficient to trigger the disease phenotype [2]. Several hepatotoxic factors have been recognized as triggers of clinical PCT in both familial-PCT and sporadic-PCT, including alcohol abuse, chronic hepatitis C virus (HCV) infection, use of oral estrogens, liver iron overload, and halogenated aromatic hydrocarbons exposure [1, 3]. Altered liver enzymes are observed in the majority of patients with PCT at presentation; a histopathological picture of chronic hepatitis and liver siderosis is observed in the majority of patients.
Tamoxifen is an antagonist of the estrogen receptor in breast tissue that has been the standard endocrine (anti-estrogen) therapy for hormone-positive breast cancer used since 1969. It represents a widely used and well-tolerated treatment for patients with all stages of breast cancer and it has a low level of adverse effects [4]. Theoretically, long-term use of tamoxifen may induce a wide spectrum of hepatic injuries, namely periportal hepatitis, cirrhosis, macrovesicular steatosis, steatohepatitis, and iron overload [5]. In 2 conventional carcinogenicity bioassays published in 1993 and 2006 [6, 7], tamoxifen was found to produce liver tumors in rats, related to a genotoxic activity. However, a trial published in 2009 [8] compared the differences in mebabolite-mediated toxicity of this drug in rats versus humans; the authors concluded that the toxic profile of tamoxifen was higher in rat than in human liver. The current understanding suggests that the hepatotoxicity of tamoxifen is evident but its toxic profile in humans still needs to be established.
Case report
 |  |
| Figure 1 | Figure 2 |
|---|
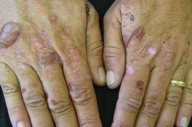
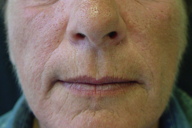
We observed a 53-year-old woman with a two year history of purplish macules, erosions, bullae, and scars on the back of the hands after minor trauma (Figure 1). She also exhibited hyperpigmentation on sun exposed areas, hypertrichosis of the face (Figure 2), and reddish coloration of the urine. Hyperpigmentation and skin fragility worsened during spring and summer. Three years prior to presentation, the patient was diagnosed an invasive ductal carcinoma of the right breast and had been treated with radical mastectomy. The procedure was followed by adjuvant radiotherapy and tamoxifen 20 mg per day. There was no prior history of exposure to other drugs, alcohol, or viral hepatitis; there were no photosensitive dermatoses family members.
 |
| Figure 3 |
|---|
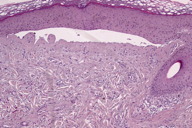
Histological examination of a hand lesion revealed a subepidermal blister, acanthosis of the epidermis, and an inflammatory infiltration surrounding ectatic vessels in the papillary dermis (Figure 3).
The laboratory evaluation at presentation showed an increase in hepatic enzymes: alanine aminotransferase (ALT) to 70 U/L (reference range (RR): 10-30 U/L), aspartate aminotransferase (AST) to 60 U/L (RR: 10-30 U/L) and ɣ-glutamyl tranferase to 130 U/L (RR: 7-32 U/L), with an ALT/AST ratio>1. Urinary porphyrin excretion analysis revealed markedly elevated levels of total porphyrins up to 6554 μg/24h (RR: <220 μg/24h), with a clear increase in the level of coproporphyrins to 5612 μg/24h (RR: <160 μg/24h), and a moderate increase of the uroporphyrins to 941 μg/24h (RR: <60 μg/24h). The levels of 5-aminolevulinic acid and porphobilinogen were within normal range. All other complementary exams, namely renal function, iron kinetics, viral serology, immunological studies, abdominal ultrasound, and thoraco-abdominal scanning were normal or negative.
Clinical, histological, and laboratory evaluation were consistent with the diagnosis of PTC. The patient was than advised to replace tamoxifen with letrozole (2.5 mg id), and to adopt sun protection measures. The hypertrichotic areas were treated with Intense Pulse Light (IPL) 640 nm.
Urinary total porphyrin levels fell from 6554 μg/24h to 2845 μg/24h by the third month and to normal levels by the sixth month after stopping tamoxifen. Liver enzymes also normalized over this period; the skin lesions did not recur. The patient is still being followed up at our department and two years after remission there are no clinical or laboratory signs of PCT.
Discussion
In this case, the temporal relationship between the symptoms and tamoxifen in a patient medicated exclusively with this drug, the progressive fall of urine porphyrin levels following the interruption of the treatment and the absence of any other causative factor of PCT all support the diagnosis of PCT induced by tamoxifen.
In addition to the case we report here, de novo occurrence of PCT during tamoxifen therapy as adjuvant medication in the treatment of breast cancer has been described in only three other cases [9, 10, 11]. However, in two of them the role of tamoxifen was uncertain because the patients were simultaneously receiving other drugs with intrinsic porphyrinogenic activity (cyclophosphamide and 5-fluorouracil or carboplatin) [9, 10].
Although sporadic PCT is often precipitated by environmental hepatotoxic factors, recent studies suggest that, in addition, there may be a genetic susceptibility, which explains why not all persons under those circumstances develop PCT [12].
The mechanism of action by which hepatotoxic factors trigger PCT is still unclear; nevertheless many authors propose possible mechanisms to explain the porphyrinogenic capability of tamoxifen. It may induce PCT because of its partial intrinsic estrogenic activity. However, the mechanism by which estrogens induce PCT is still unknown and the onset of PCT can range from 6 months to 10 years from the start of estrogen intake [3]. This latency period is consistent with the delay in onset of the symptoms in our patient (about a year). It has also been postulated that tamoxifen has a direct hepatotoxic effect. Tamoxifen is metabolized in the liver into N-demethylate metabolites by the cytochrome P450 isoenzyme, CYP3A4. The metabolites created by this reaction can bind to liver microsomes, damaging proteins and DNA in a permanent way [13]. Theoretically these metabolites can cause a partial inactivation of the UROD inducing PCT. Corroborating this are a few cases reported of non-alcoholic steatohepatitis associated with tamoxifen therapy [14]. In our case, the high levels of liver enzymes with ALT/AST ratio>1 and the normalization after tamoxifen cessation also give strength to this hypothesis. Another possible but not fully understood mechanism is liver iron overload induced by the long-term tamoxifen treatment [5].
This case highlights the importance of a detailed history and the need of a high index of suspicion concerning this apparently very rare, but significant, complication of tamoxifen. Clinicians should be aware of the porphyrinogenic capability of tamoxifen so that prompt measures can be taken. These would include replacement of the drug, photoprotection, and appropriate diet. Some authors strongly recommend regular monitoring of the liver enzymes in breast cancer patients when using tamoxifen.
References
1. Elder GH. Porphyria cutanea tarda. Semin Liver Dis. 1998; 18:67-75. [PubMed]2. Kappas A, Sassa S, Galbraith RA, Nordmann Y. The porphyrias. In: Scriver CR, Beaudet AL, Sly WS, Valle D, eds. The Metabolic and Molecular Basis of Inherited Disease. Vol II, 7th edition. New York, NY: McGraw-Hill; 1995: 2103-60.
3. Bulaj ZJ, Phillips JD, Ajioka RS, Franklin MR, Griffen LM, Guinee DJ, Edwards CQ, Kushner JP. Hemochromatosis genes and other factors contributing to the pathogenesis of porphyria cutanea tarda. Blood. 2000; 95: 1565-71. [PubMed]
4. Jordan VC. Overview from the International Conference on Long-Term Tamoxifen Therapy for Breast Cancer. J Natl Cancer Inst. 1992; 19; 84: 231-4. [PubMed]
5. Jatobá CA, de Rezende AA, de Paiva Rodrigues SJ, de Almeida Câmara MM, das Graças Almeida M, Freire-Neto F, da Rocha LR, da Medeiros AC, Brandão-Neto J, de Carvalho Formiga MC, de Azevedo IM, de Oliveira Ramos AM. Liver iron overload induced by tamoxifen in diabetic and non-diabetic female Wistar rats. Biometals. 2008;21(2): 171-8. [PubMed]
6. Greaves P, Goonetilleke R, Nunn G, Topham J, Orton T. Two-year carcinogenicity study of tamoxifen in Alderley Park Wistar-derived rats. Cancer Res. 1993; 53:3919-24. [PubMed]
7. Hashiba M, Kasahara T, Kim SY, Shibutani S, Degawa M. DNA damage and altered gene expression of enzymes for metabolism and DNA repair by tamoxifen and toremifene in the female rat liver. Cancer Sci. 2006; 97(6): 468-77. [PubMed]
8. Zhao L, Krishnan S, Zhang Y, Schenkman JB, Rusling JF. Differences in metabolite-mediated toxicity of tamoxifen in rodents versus humans elucidated with DNA/microsome electro-optical arrays and nanoreactors. Chem Res Toxicol. 2009; 22(2):341-7. [PubMed]
9. Malina L, Michalíková H, Hussarová L. Porphyria cutanea tarda after antineoplastic drugs. Cas Lek Cesk. 1999; 138:536-8. [PubMed]
10. Claus A, Gail P. Tamoxifen and porphyria. Millenium Meeting on porphyrins and Porphyria. p33 (abstract).
11. Agarwal R, Peters TJ, Coombes RC, Vigushin DM. Tamoxifen-related porphyria cutanea tarda. Med Oncol. 2002; 19:121-3. [PubMed]
12. Elder GH. Porphyria cutanea tarda: a multifactorial disease. In Recent advances in Dermatology. Vol 8. Churchill Hill Ed. Edinburgh. 1990; 55-69.
13. White, J., et al. Species differences in the covalent binding of [14C] tamoxifen to liver microsomes and the forms of cytochrome P450 involved. Biochem Pharmacol. 1995; 49:1035-1042. [PubMed]
14. James, O. and Day, C. Non-alcoholic steatohepatitis (NASH): a disease of emerging importance. J. Hepatol. 1998; 29:495-501. [PubMed]
© 2010 Dermatology Online Journal

