Granulomatous lesion on the face successfully treated with antitubercular therapy
Published Web Location
https://doi.org/10.5070/D38kv8h7hwMain Content
Letter: Granulomatous lesion on the face successfully treated with antitubercular therapy
José M Ricart1, José M Martín2, Ana Renau3, Jaide Pérez4
Dermatology Online Journal 16 (9): 13
1. Dermatology Department, Hospital La Fe, Valencia, Spain. jricartv@yahoo.es2. Dermatology Department, Hospital Clinico Universitario, Valencia, Spain
3. Internal Medicine Department, Hospital la Fe, Valencia, Spain
4. Pathology Department, Hospital la Fe, Valencia, Spain
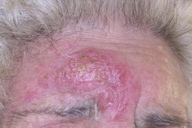
An 84-year-old woman, who presented with a one-year history of a persistent and infiltrated plaque on the forehead, was referred to our dermatology clinic. Upon clinical examination a 2.2 cm x 3 cm plaque with an erythematous, scaly, violaceous, and elevated border was seen (Figure 1).
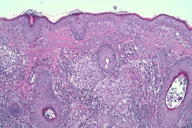
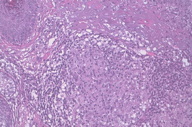
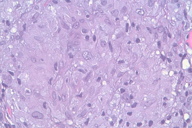
Punch biopsies showed multiple epithelioid cell granulomata without necrosis in the reticular dermis, with a moderate peripheral lymphocytic infiltration. No significant epidermal lesions were present. Dermal epithelioid cell granuloma with peripheral lymphocytic component and absence of necrosis (Figures 2 through 4).
Biochemical and hematological laboratory investigations were normal except for an increased sedimentation rate (50 mm/h) and fibrinogen (471 mg/dl). Chest X-ray showed no abnormalities. Serology for syphilis and human immunodeficiency virus were negative. A tuberculin test (PPD), performed twice, was negative. Cultures from the lesion, sputum, and urine for bacteria, fungi, atypical mycobacteria, and Mycobacterium tuberculosis were repeated four times, but all were negative. New biopsies were performed three times showing similar characteristics mentioned above. In addition, polymerase chain reaction (PCR) did not detect mycobacterial DNA.
 |
| Figure 5 |
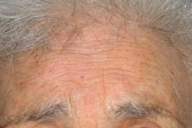
|---|
| Figure 5. Resolution of the lesion after tuberculostatic treatment. |
The patient began treatment with topical corticosteroids but the lesion did not improve. Empirical antitubercular therapy (isoniazid 50 mg/d, pyrazinamide 300 mg/d, rifampicin 120 mg/d, and ethambutol 400 mg/d) was administered for six months. The plaque progressively resolved (Figure 5).
In this patient, mycobacteria were not demonstrated in skin biopsies or tissue cultures and the polymerase chain reaction was also negative.
Nevertheless, a therapeutic trial of antitubercular treatment was initiated with a successful response. Diagnosing granulomatous skin disease is very difficult in some cases. In these cases, a trial of antitubercular therapy seems warranted for both diagnostic and therapeutic purposes when cutaneous tuberculosis or atypical mycobacterial infection is suspected.
Lupus vulgaris is a chronic and progressive form of secondary cutaneous tuberculosis. Clinically, this condition is characterized by soft reddish-brown plaques. The lesions pursue a chronic course over several years and grow by peripheral extension and central scarring [1]. The diverse clinical forms of lupus vulgaris include papular, nodular, plaque, ulcerative, vegetative, and tumor-like lesions. Head and neck are the predominant sites affected [2, 3, 4].
Atypical localizations and atypical clinical presentations of lupus vulgaris may occur and lead to difficulty in diagnosis and treatment [5]. The clinical presentation of cutaneous tuberculosis may vary depending upon host immunity, infection route, and previous exposure. Atypical clinical forms, such as cellulitis, folliculitis, lichen simplex chronicus, sporotrichoid, framboesiform, lichenoid gangrenous, ulcero-vegetant, and tumor-like types may occur. These clinical forms of lupus vulgaris emerge sporadically and often lead to misdiagnosis and delayed or incorrect therapy. In our case, the infiltrated and slightly ulcerated plaque simulated focal mucinosis, lymphoma, sarcoidosis, leishmaniasis, or mycobacterial disease.
Leishmaniasis, characterized by cutaneous ulceration and involvement of oro-nasal mucosa, may progress to destruction of the central structures of the face including nose. Histopathology shows a granulomatous infiltrate and Leishman-Donovan bodies representing the parasites, which can be grown on culture. Syphilis, another chronic condition, should be ruled out by serology and special histological findings. Lymphoma, focal mucinosis, and sarcoidosis were also excluded on the basis of histopathology and the progression in spite of corticosteroid treatment. The diagnosis of cutaneous tuberculosis is normally made by clinical and histopathological data. Laboratory methods such as PPD test, culture of bacilli, PCR, and MycoDot serology tests are supportive of the diagnosis [6, 7]. However, because most types of cutaneous tuberculosis are paucibacillary, it is often difficult to demonstrate or grow the organism. Serological tests have also not proved to be very useful, especially in endemic areas.
Polymerase chain reaction (PCR) based on detection of Mycobacterium tuberculosis DNA in skin samples may extend and improve the diagnostic panel for cutaneous tuberculosis and may be also use to differentiate atypical mycobacterial infections, especially in cases with negative cultures [8, 9]. Although specific, some studies have demonstrated only up to 75 percent sensitivity of the technique with the common varieties of cutaneous tuberculosis [10, 11]. However, some authors did not find it useful in paucibacillary forms [8].
Several authors have found that a therapeutic trial of antitubercular drugs is helpful in confirming or refuting the diagnosis of cutaneous tuberculosis in difficult cases, especially when other tests do not provide clear answers [11, 12, 13]. One study showed that 91 percent of patients, in whom a diagnosis of cutaneous tuberculosis was suspected, responded to empiric therapy. The authors concluded that patients who did not respond to therapy did not have tuberculosis [12].
In this case, the absence of an alternative plausible diagnosis and the rapid response to an antitubercular therapeutic trial confirmed the diagnosis of cutaneous mycobacteriosis. Although the most plausible diagnosis was lupus vulgaris, we could not confirm the diagnosis because all laboratory tests performed were negative and the drug regimen could also be effective for other atypical mycobacterial infections.
In conclusion, diagnosing granulomatous skin disease is very difficult in some cases. A trial of antitubercular therapy seems warranted for both diagnostic and therapeutic purposes when cutaneous mycobacteriosis is suspected and cannot be confirmed.
References
1. Kumar B, Muralidhar S. Cutaneous tuberculosis: a twenty year prospective study. Int J Tuberc Lung Dis. 1999; 3: 494-500. [PubMed]2. Sehgal VN, Wagh SA. Cutaneous tuberculosis. Current concepts. Int J Dermatol. 1990; 29: 237-52. [PubMed]
3. Kanwar AJ. Lupus vulgaris following BCG vaccination. Int J Dermatol. 1988; 27: 525-6. [PubMed]
4. Khandpur S, Nanda S, Reddy BSN. An unusual episode of lupus vulgaris masquerading as sporotrichosis. Int J Dermatol. 2001; 40: 336-9. [PubMed]
5. Khandpur S, Reddy BSN. Lupus vulgaris: unusual presentations over the face J Eur Acad Dermatol Venereol. 2003; 17: 706-10. [PubMed]
6. Rao L, Padmavathy LRL. Utility of Mycodot test in the diagnosis of cutaneous tuberculosis. Indian J Dermatol Venereol Leprol 2003; 69: 428-9. [PubMed]
7. Maroto MC, Bettinardi A, et al. Diagnosis of cutaneous tuberculosis in biopsy specimens by PCR and southern blotting. J Clin Pathol. 1996; 49: 889-91. [PubMed]
8. Padmavathy L, Lakshmana Rao L, Veliath AJ, et al. Utility of polymerase chain reaction as a diagnostic tool in cutaneous tuberculosis. Indian J Dermatol Venereol Leprol 2003; 69: 214-6. [PubMed]
9. Dundar D, Sayan M, Arsian Z, Tamer GS, Dundar V. Routine using pattern and performance of diagnostic tests for tuberculosis on a university hospital. Am J Med Sci 2010; 339: 244-8. [PubMed]
10. Sehgal VN, Sardana K, Sehgal R, Sharma S. The use of anti-tubercular therapy (ATT) as a diagnostic tool in pediatric cutaneous tumerculosis. Int J Dermatol 2005; 44: 961-3. [PubMed]
11. Ramam M, Mittal R, Ramesh V. How soon does cutaneous tuberculosis respond to treatment? Implications for a therapeutic test of diagnosis. Int J Dermatol 2005; 44: 121-4. [PubMed]
12. Ramam M, Ramesh V. Trial of antitubercular therapy in the diagnosis of cutaneous tuberculosis. Am J Dermatopathol 2010; 32: 316. [PubMed]
13. Tan SH, Tan BH, Goh CL, et al. Detection of M. tuberculosis DNA using PCR in cutaneous tuberculosis and tuberculides. Int J Dermatol 1999; 38: 122-7. [PubMed]
© 2010 Dermatology Online Journal