Linear IgA bullous dermatosis following influenza vaccination
Published Web Location
https://doi.org/10.5070/D37ff467xpMain Content
Linear IgA bullous dermatosis following influenza vaccination
Lauren Alberta-Wszolek MD1, Alyse M Mousette BS2, Meera Mahalingam MD PhD FRC Path, Nikki A Levin MD PhD4
Dermatology Online Journal 15 (11): 3
1. Assistant Professor of Dermatology, Division of Dermatology, Department of Medicine, UMass Memorial Healthcare, Worcester,
Massachusetts2. University of Massachusetts Medical School, Worcester, Massachusetts
3. Dermatopathology Section, Department of Dermatology, Boston University School of Medicine, Boston, Massachusetts
4. Associate Professor of Dermatology, Division of Dermatology, Department of Medicine, UMass Memorial Healthcare, Worcester, Massachusetts. nikki.levin@umassmemorial.org
Abstract
Linear IgA Bullous Dermatosis (LABD) is an immune-mediated subepidermal vesiculobullous eruption characterized by linear deposits of IgA at the basement membrane zone. Most cases are idiopathic but medications, infections, and malignancies have also been reported to induce LABD. We report the case of a 54-year-old woman who developed LABD shortly after receiving an influenza vaccination.
Introduction
Linear IgA Bullous Dermatosis (LABD) is an immune-mediated subepidermal blistering disease characterized by linear deposits of IgA along the basement membrane zone. The clinical presentation is variable and patients can manifest findings suggestive of dermatitis herpetiformis as well as subepidermal tense bullae often indistinguishable from bullous pemphigoid. Patients often present with pruritic annular or grouped papules, vesicles, and bullae distributed symmetrically on extensor surfaces. Mucosal involvement may also be a prominent feature. Different clinical manifestations are likely the result of varying antigenic targets within the basement membrane zone. Most cases are idiopathic but medications, infections, and malignancies have all been reported as potential inducers of this condition.
In this report, we present a case of LABD that began 2 days after a woman received an influenza vaccination.
Case report
A 54-year-old healthy Caucasian female presented with a two-week history of a severely pruritic, vesiculobullous eruption on her legs, arms and lower abdomen that developed two days after she had received an influenza vaccination intramuscularly. The rash began as tiny pink papules that then formed vesicles. She continued to get new lesions despite taking diphenydramine and cetirizine as prescribed by her primary care physician. She had not tried topical corticosteroids or any other treatments. She denied changes in her medications, use of herbal supplements or other over-the-counter medications. She denied fever, chills, upper respiratory or gastrointestinal symptoms, or mucous membrane involvement.
Her past medical history was significant for osteoporosis, hyperlipidemia and depression. Her medications were alendronate, calcium, vitamin D, rosuvastatin, and sertraline at the time her eruption began. The only recent change in her medications was substitution of generic sertraline for brand name Zoloft, which had occurred two months prior to the eruption. Otherwise, she had been on rosuvastatin for five months and her other medications for over one year. She discontinued rosuvastatin and switched back to brand name Zoloft with no improvement.
 |  |
| Figure 1 | Figure 2 |
|---|---|
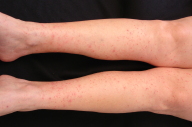
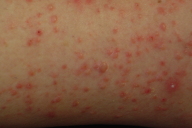
| Figure 1. Symmetric and bilateral discrete pink papules and vesicles on lower legs, with concentration over medial calves. Figure 2. Close-up view of pink papules and vesicles. | |
Physical examination revealed symmetrically distributed discrete, pink papules, vesicles and bullae over her lower extremities, hands, forearms, and lower abdomen with particular concentration on the medial surfaces of her calves (Figs. 1 & 2). She had no conjunctival injection, involvement of mucous membranes, palms or soles.
 |  |
| Figure 3 | Figure 4 |
|---|---|
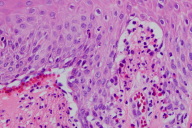
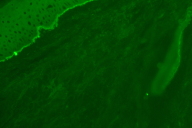
| Figure 3. Subepidermal blister with neutrophils Figure 4. Linear IgA deposits along basement membrane zone on direct immunofluorescence | |
Microscopic examination of a biopsy from the left calf revealed a subepidermal blistering process with neutrophils (Fig. 3). Direct immunofluorescence revealed linear deposits of immunoglobulin A (negative IgG, IgM, C3, and fibrinogen) along the basement membrane zone (Fig. 4). These findings in conjunction with the histology were most suggestive of LABD.
Routine laboratory tests were normal including complete blood count and antiendomysial antibodies.
The eruption cleared after two courses of prednisone followed by a mid-potency topical corticosteroid and did not recur when she restarted generic sertraline and rosuvastatin. The eruption lasted six weeks in total.
Discussion
There are many reports in the literature of medications eliciting LABD, most notably vancomycin [1, 2, 3], but also several others including penicillins, cephalosporins, ACE inhibitors and non-steroidal anti-inflammatories [4]. Typically, the lesions of drug-induced LABD arise within 7 to 14 days of onset of the offending medication and resolve within a few weeks after discontinuation of the drug [1, 2, 5]. The eruption often returns with rechallenge [6].
To our knowledge, this is the first report of LABD associated with the influenza vaccination. Linear IgA Bullous Dermatosis may be idiopathic, but given the temporal association between the influenza vaccination and onset of the eruption, we feel the immunization was the likely trigger. Our patient's medications, namely alendronate, calcium, vitamin D, sertraline and rosuvastatin, have never been reported in association with LABD. One case report cited atorvastatin as a cause of LABD in a patient who began the medication two weeks prior to development of the eruption [7]. However, in our case, the patient's medications are unlikely culprits as she had been on them for many months when the eruption began. Furthermore, the eruption resolved while she continued to take alendronate, calcium and vitamin D and did not recur when she restarted generic sertraline and rosuvastatin. Thus, it seems plausible that the influenza vaccination prompted development of LABD in this patient. Although a rechallenge with influenza vaccination followed by redevelopment of LABD would provide stronger evidence for a causal relationship, we did not feel that it would be medically advisable for the patient to take this risk, and she has not received further influenza vaccinations.
Influenza vaccinations have been reported to trigger autoimmune phenomena. In particular, several reports of new onset bullous pemphigoid [8, 9, 10, 11] and exacerbation of preexisting pemphigoid [10, 12] have been temporally associated with influenza immunization. The time period of latency from vaccination to disease onset ranged from one day to less than one month in all these cases [8, 9, 10, 11, 12]. However, although cases do occur, the likelihood of influenza vaccination triggering severe bullous pemphigoid on a large scale is rare [13]. Another example of induction of autoimmune dermatologic disease after influenza vaccination is in a recent publication by De Simone et al. [14]. This group described a patient with pemphigus vulgaris in remission who developed recurrence of erosions and bullous lesions 7 days after influenza immunization. In retrospect, he had a similar reactivation years before, again 7-10 days following influenza vaccination [14]. Besides dermatologic manifestations, other organ systems have been affected by autoimmune conditions following influenza vaccination, in particular neurologic, rheumatic, hematologic, and cardiac [15]. The majority of these manifestations occurred within 4 weeks of vaccination, and reactions were usually self-limited [15]. The exact mechanism of autoimmune reactions following vaccination is not entirely clear. Possible explanations include molecular mimicry, in which a viral antigen has structural similarity to a host-antigen [16], direct or indirect activation of the host's immune system by viral antigens or cytokines, respectively [15], or even other components of the vaccine that may be noxious to the host [15].
Multiple antigenic targets exist in idiopathic LABD such as the 97-kDa extracellular domain of BPAg2 [17], the 120-kDa ectodomain of BPAg2 [18, 19], LAD285 [20, 21], BP230 [20], and type VII collagen [22] as well as others. Drug-induced LABD appears to have even more heterogeneous target antigens [23]. Because these target proteins are important adhesive and structural components of the basement membrane zone, autoantibody attack on these antigens results in separation of the layers, or blister formation. In an ideal world, the antigenic target may determine the clinical phenotype. However, it is becoming more apparent that LABD is notable for heterogeneous clinical and immunopathologic features [24, 25, 26, 27]. In conclusion, for our patient, it is likely that the influenza vaccination triggered her disease. Our patient fits the clinical picture of drug-induced LABD given the temporal association of the eruption, lack of mucosal involvement, and self-limited course. The drug, in her case, was the influenza vaccination triggering LABD.
References
1. Carpenter S, Berg D, Sidhu-Malik N, Hall RP III, Rico MJ. Vancomycin-associated linear IgA dermatosis: a report of three cases. J Am Acad Dermatol 1992;26:45-8. [PubMed]2. Kuechle MK, Stegemeir E, Maynard B, Gibson LE, Leiterman KM, Peters MS. Drug-induced linear IgA bullous dermatosis. J Am Acad Dermatol 1994;30:187-92. [PubMed]
3. Whitworth JM, Thomas I, Peltz SA, Sullivan BC, Wolff AH, Cytryn AS. Vancomycin induced linear IgA bullous dermatosis (LABD). J Am Acad Dermatol 1996;34:890-1. [PubMed]
4. Hull CM, Zone JJ. Dermatitis herpetiformis and linear IgA bullous dermatosis. In: Bolognia J, Jorizzo J, Rapini R, editors. Dermatology, 2nd ed. Mosby Elsevier; 2008. p. 447-56.
5. Nousari HC, Kimyai-Asadi A, Caeiro JP, Anhalt GJ. Clinical, demographic, and immunohistologic features of vancomycin-induced linear IgA bullous disease of the skin: report of 2 cases and review of the literature. Medicine (Baltimore) 1999;78:1-8. [PubMed]
6. Neughebauer BI, Negron G, Pelton S, Plunkett RW, Beutner EH, Magnussen R. Bullous skin disease: an unusual allergic reaction to vancomycin. Am J Med Sc. 2002;323:273-8. [PubMed]
7. König C, Eickert A, Scharfetter-Kochanek K, Krieg T, Hunzelmann N. Linear IgA bullous dermatosis induced by atorvastatin. J Am Acad Dermatol 2001;44:689-92. [PubMed]
8. Fournier B, Descamps V, Bouscarat F, Crickx B, Belaich S. Bullous pemphigoid induced by vaccination. Br J Dermatol 1996;135:153-4. [PubMed]
9. Lear JT, Tan BB, English JS. Bullous pemphigoid following influenza vaccination. Clin Exp Dermatol 1996;21:392. [PubMed]
10. Downs AM, Lear JT, Bower CP, Kennedy CT. Does influenza vaccination induce bullous pemphigoid? A report of four cases. Br J Dermatol 1998;138:363. [PubMed]
11. Nikkels AF, Nikkels-Tassoudji N, Piérard GE. Cutaneous adverse reactions following anti-infective vaccinations. Am J Clin Dermatol 2005;6:79-87. [PubMed]
12. Bodokh I, Lacour JP, Bourder JF, Rodot S, Botcazou V, Ortonne JP. Réactivation de pemphigoϊde bulleuse après vaccination anti-grippale. Therapie 1994;49:154. [PubMed]
13. Garcia-Doval I, Mayo E, Noguiera Farina J, Cruces MJ. Bullous pemphigoid triggered by influenza vaccination? Ecological study in Galicia, Spain. Br J Dermatol 2006;155:820-3. [PubMed]
14. De Simone C, Caldarola G, D'agostino M, Zampetti A, Amerio P, Feliciani C. Exacerbation of pemphigus after influenza vaccination. Clin Exp Dermatol 2008;33:1-3. [PubMed]
15. Schattner A. Consequence or coincidence? The occurrence, pathogenesis and significance of autoimmune manifestations after viral vaccines. Vaccine 2005;23:3876-86. [PubMed]
16. Schoenfeld Y, Aron-Maor A. Vaccination and autoimmunity- 'Vaccinosis': A dangerous liaison?. J Autoimmune. 2000;14:1-10. [PubMed]
17. Zone JJ, Taylor TB, Kadunce DP, Chorzelski TP, Schachner LA, Huff JC et al. IgA Antibodies in chronic bullous diseases of childhood react with 97-kDa basement membrane zone protein. J Invest Dermatol 1996;106:1277-80. [PubMed]
18. Roh JY, Yee C, Lazarova Z, Hall RP, Yancey KB. The 120-kDa soluble ectodomain of type XVII collagen is recognized by autoantibodies in patients with pemphigoid and linear IgA dermatosis. Br J Dermatol 2000;143:104-11. [PubMed]
19. Schumann H, Baetge K. Tasanen K, Wojnarowska F, Schacke H, Zillikens D et al. The shed ectodomain of collagen XVII/BP180 is targeted by autoantibodies in different blistering skin diseases. Am J Pathol 2000;156:685-95. [PubMed]
20. Allen J, Wojnarowska F. Linear IgA disease: the IgA and IgG response to the epidermal antigens demonstrates that intermoleculat epitope spreading is associated with IgA rather than IgG antibodies, and is more common in adults. Br J Dermatol 2003;149:977-85. [PubMed]
21. Allen J, Wojnarowska F. Linear IgA disease: the IgA and IgG response to dermal antigens demonstrates a chiefly IgA response to LAD285 and a dermal 180-kDa protein. Br J Dermatol 2003;149:1055-8. [PubMed]
22. Hashimoto T, Ishiko A, Shimizu H, Tanaka T, Dodd HJ, Bhogal BS et al. A case of linear IgA bullous dermatosis with IgA and anti-type VII collagen autoantibodies. Br J Dermatol 1996;134:336-9. [PubMed]
23. Paul C, Wolkenstein P, Prost C, Caux F, Rostoker G, Heller M et al. Drug-induced linear IgA disease: target antigens are heterogeneous. Br J Dermatol 1997;136:406-11. [PubMed]
24. Sobjanek M, Sokolowska-Wojdylo M, Sztaba-Kania M, Barañska-Rybak W, Maciejweska A and Wlodarkiewicz A. Clinical and immunopathological heterogeneity of 22 cases of linear IgA bullous dermatosis. J Eur Acad Dermatol Venereol 2008;22:1131. [PubMed]
25. Wojnarowska F, Marsden RA, Bhogal B, Black MM. Chronic bullous disease of childhood, childhood cicatricial pemphigoid, and linear IgA disease of adults. A comparative study demonstrating clinical and immunopathologic overlap. J Am Acad Dermatol 1988;19:792-805. [PubMed]
26. Sokolowska-Wojdylo M, Sztaba-Kania M, Sobjanek M, Barañska-Rybak W. Heterogenity of clinical features of linear IgA bullous dermatosis (LABD) in the materials of Laboratory of Immunodermatology and Serology in Dermatological Department Medical University of Gdañsk. Post Derm Alergol 2007;24:82-8.
27. Chorzelski TP, Jabloñska S, Maciejowska E. Linear IgA bullous dermatosis of adults. Clin Dermatol 1991;9:383-92. [PubMed]
© 2009 Dermatology Online Journal

