Terbinafine-induced acute generalized exanthematous pustulosis (AGEP) responsive to high dose intravenous corticosteroid
Published Web Location
https://doi.org/10.5070/D375c2w9p2Main Content
Terbinafine-induced acute generalized exanthematous pustulosis (AGEP) responsive to high dose intravenous corticosteroid
Omar A Ibrahimi1, Nilanthi Gunawardane2, Alireza Sepehr3, Rachel V Reynolds4
Dermatology Online Journal 15 (9): 8
1. Harvard Medical School, Department of Dermatology, Massachusetts General Hospital, Boston, Massachusetts2. Harvard Medical School, Boston, Massachusetts
3. Harvard Medical School, Department of Pathology, Beth Israel Deaconess Medical Center, Boston, Massachusetts
4. Harvard Medical School, Department of Dermatology, Beth Israel Deaconess Medical Center, Boston, Massachusetts. rreynold@bidmc.harvard.edu
Abstract
Acute generalized exanthematous pustulosis (AGEP) is a febrile pustular drug eruption. Terbinafine, an allylamine fungicidial agent, is the most common anti-mycotic associated with AGEP. Here, we report a case of terbinafine-induced AGEP that was recalcitrant to oral corticosteroid but responsive to high-dose intravenous corticosteroid.
Case report
A 47-year-old woman with no significant past medical history presented to our outpatient clinic with a four-day history of a symmetric, pruritic, erythematous rash on her trunk and extremities that developed six days after starting oral terbinafine 250 mg daily for onychomycosis. It began on her trunk and spread quickly over the previous two days to her extremities. On physical exam, she had 0.5-1.5 cm erythematous targetoid plaques with dusky centers, coalescing in a polycyclic and annular arrangement over 30-40 percent of her total body surface area. There were a total of five to ten pustules overlaying some of her targetoid lesions. The palms, soles, mouth, eyes, nasal mucosa and vaginal mucosa were spared. She stated she was extremely bothered by her rash and was irate at the nurse practitioner who initially provided the prescription, for "blowing her off" when she called to complain about her rash. Review of systems was negative for fever, chills, dysuria, dysphagia, and skin pain, but notable for increased lethargy and depression. To her knowledge she had never previously been exposed to terbinafine, nor did she have a past or family history of psoriasis. The differential diagnosis consisted of exanthematous drug eruption, erythema multiforme (EM), Stevens-Johnson syndrome (SJS), and acute generalized exanthematous pustulosis (AGEP). A 4 mm punch biopsy was obtained for clinicopathological correlation. The patient was instructed to immediately discontinue the terbinafine. Given her distress, she was also started on oral prednisone 60 mg daily, topical clobetasol twice daily under occlusion and diphenhydramine 25-50 mg by mouth every four to six hours as needed for itch.
 |
| Figure 1 |
|---|
| Figure 1. Erythematous atypical targetoid plaques studded with numerous overlaying non-follicular pustules |
A follow-up phone call to the patient was placed on the following day and the patient noted fading of her lesions, as well as a halting in the progression of her rash. She also noted improved energy and mood. However, the patient returned to clinic two days after her initial visit with a complaint of skin pain, progression of her rash to her extremities and subjective fevers. On repeat physical exam, she now had erythematous atypical targetoid plaques studded with numerous overlaying non-follicular pustules (Fig. 1), involving 70 percent of her total body surface area. A few flaccid bullae were seen on her thighs and buttocks accounting for approximately 1 percent detachment of her body surface area. Again, no mucosal lesions were noted and Nikolsky sign was negative. Given the worsening of her rash, development of skin pain, as well as bullae formation, the patient was hospitalized due to concern for progressing SJS/Toxic epidermal necrolysis (TEN). Repeat biopsies for histology and culture were obtained, basic labs were drawn, and cultures were also taken from her pustules. She was started on intravenous high-dose corticosteroid (methylprednisolone 2.4 mg/kg for total dose of 125 mg daily), antipyretics (acetaminophen 650 mg by mouth every four hours as needed for fever), antipruritics (diphenhydramine 25-50 mg by mouth every four to six hours as needed for itch), vigilant wound care (Petrolatum-impregnated gauze dressings to denuded areas daily), and close observation (vital signs monitored every six to eight hours).
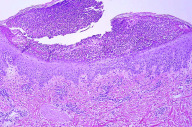 |
| Figure 2 |
|---|
| Figure 2. Histopathologically, spongiosis, sub-corneal pustule, superficial perivascular lympho-histiocytic and eosinophilic infiltrate with occasional dermal eosinophils (H&E, x20) |
Her complete blood count was significant for a white blood cell count of 19,700 per microliter with 89.0 percent neutrophils and 0 percent eosinophils. Renal and liver function tests were within normal limits. Bacterial swabs obtained from the pustules were negative and her cultures were unremarkable. The biopsy obtained at the initial clinic visit revealed spongiosis and sub-corneal pustules, with superficial perivascular lympho-histiocytic and eosinophilic infiltrate and occasional dermal eosinophils, consistent with AGEP (Fig. 2). Subsequent biopsy specimens were also consistent with AGEP. Periodic Acid-Schiff (PAS) was negative. The rash improved dramatically within 36 hours of intravenous methylprednisolone with decreased erythema, edema, and extensive desquamation. She tolerated the intravenous corticosteroids without any complications and received a total of three doses of intravenous methylprednisolone. She was then transitioned to oral prednisone 120 mg daily and discharged with a twenty-four day oral prednisone taper. The prednisone taper was devised as follows, three days of 120 mg oral prednisone followed by a incremental decrease in dose by 20 mg every third day until the patient completed 20 mg of oral prednisone for three days; the patient then took 10 mg of oral prednisone for three days followed by 5 mg of oral prednisone for three days and then discontinued the medication completely. She was also placed on GI prophylaxis (Pantoprazole 40 mg by mouth daily) and daily calcium (500 mg) and vitamin D (400 units) supplementation for the duration of her prednisone taper. Her erythema and edema completely resolved, with minimal post-inflammatory hyperpigmentation, within one week following discharge. A follow-up exam three weeks after discharge revealed complete resolution of her rash without sequelae. There was no recrudescence of her rash during or after the prednisone taper.
Discussion
A form of acute pustular eruption related to medications was first described by Baker and Ryan in 1968 [1]. However, it was in 1980 that Beylot et al. introduced the term acute generalized exanthematous pustulosis to describe this entity [2]. Following a retrospective analysis of 63 patients with AGEP, Roujeau et al. described 5 criteria for the diagnosis of AGEP. These include: 1) numerous, non-follicular pustules (<5 mm) arising on widespread edematous erythema, 2) histopathology showing subcorneal and/or intraepidermal pustules often with dermal edema and perivascular infiltrates of neutrophils or eosinophils, 3) fever >38˚C, 4) blood neutrophils greater than 7 x 109/L, and 5) acute onset with spontaneous resolution of pustules in less than 15 days [3].
Subsequently, a comprehensive validation score for AGEP was devised by Sidoroff et al. based on a multinational epidemiological case-control study on severe cutaneous adverse reactions (EuroSCAR) [4]. A score is assigned based on morphological criteria (presence of pustules and erythema, distribution of lesions, postpustular desquamation), histological criteria (presence of subcorneal and/or intraepidermal pustules, spongiosis, papillary edema), and disease course (mucosal involvement, acute onset, time to resolution, fever, neutrophil count). A score of 8-12 points is needed for the definitive diagnosis of AGEP [4]. Our patient receives a score of at least 10 points for the compatible distribution and presence of pustules, erythema, post-pustular desquamation, fever, and characteristic histopathology on this AGEP validation score of the EuroSCAR study group.
Medications most commonly linked to the development of AGEP include aminopenicillins and macrolides [4, 5]. The EuroSCAR study revealed that pristinamycin, ampicillin/amoxicillin, quinolones, chloroquine, sulfonamides, terbinafine, and diltiazem were also associated with the development of AGEP [5]. This study also revealed that the causative agents for SJS/TEN and AGEP are not identical. Non-antibiotic medications that commonly trigger SJS/TEN such as allopurinol and antiepileptics did not appear to be highly associated with the development of AGEP. Terbinafine, a fungicidal allylamine, is the most common antimycotic agent associated with AGEP [6]. To date, there have been at least 15 cases of AGEP induced by terbinafine reported in the literature [6, 7, 8, 9]. Although rare, terbinafine has been reported to cause EM/SJS/TEN as well [10, 11].
Discontinuation of the offending agent and symptomatic treatment are generally agreed upon in the treatment of AGEP. Systemic corticosteroids have been utilized for treatment of terbinafine-induced AGEP in the majority of cases described in the literature, but the benefit remains unclear [7]. Bajaj et al. reported the development of AGEP in a woman on chronic low doses of oral corticosteroids for bullous pemphigoid [7]. Furthermore, in their review of the literature they observed that the time to resolution with corticosteroids is greater than the time to resolution without corticosteroids. Interestingly, it is unclear whether the amount of corticosteroid given or the relative severities of corticosteroid-treated cases of AGEP were confounding factors. Because the mortality of AGEP can approach up to 5 percent [12], and our patient's rash was progressing with the development of skin pain, we decided to treat with high-dose intravenous corticosteroids. Our rationale behind this stemmed from a recent uncontrolled case series of SJS/TEN patients suggesting that pulse high dose corticosteroid therapy may be more efficacious than low dose corticosteroid therapy [13]. It is important to note, however, that this study did not approach statistical significance due to its small sample size and that it still remains unclear if corticosteroids of any dosage are of benefit in SJS/TEN. Intriguingly, our patient's rash markedly improved within 36 hours of starting intravenous methylprednisolone, thereby suggesting that a short course of high-dose intravenous corticosteroids may also be of benefit in severe cases of AGEP. Further studies would be required to validate this observation.
References
1. Baker H, Ryan TJ. Generalized pustular psoriasis. A clinical and epidemiological study of 104 cases. Br J Dermatol. 1968 Dec;80(12):771-93. [PubMed]2. Beylot C, Bioulac P, Doutre MS. Acute generalized exanthematic pustuloses (four cases) (author's transl). Ann Dermatol Venereol. 1980 Jan-Feb;107(1-2):37-48. [PubMed]
3. Roujeau JC, Bioulac-Sage P, Bourseau C, et al. Acute generalized exanthematous pustulosis. Analysis of 63 cases. Arch Dermatol. 1991 Sep;127(9):1333-8. [PubMed]
4. Sidoroff A, Halevy S, Bavinck JN, et al. Acute generalized exanthematous pustulosis (AGEP)--a clinical reaction pattern. J Cutan Pathol. 2001 Mar;28(3):113-9. [PubMed]
5. Sidoroff A, Dunant A, Viboud C, et al. Risk factors for acute generalized exanthematous pustulosis (AGEP)-results of a multinational case-control study (EuroSCAR). Br J Dermatol. 2007 Nov;157(5):989-96. Epub 2007 Sep 13. [PubMed]
6. Beltraminelli HS, Lerch M, Arnold A, et al. Acute generalized exanthematous pustulosis induced by the antifungal terbinafine: case report and review of the literature. Br J Dermatol. 2005 Apr;152(4):780-3. [PubMed]
7. Bajaj V, Simpson N. Oral corticosteroids did not prevent AGEP due to terbinafine. Acta Derm Venereol. 2006;86(5):448-9. [PubMed]
8. Gréco M, Plantin P. Acute generalized exanthematous pustulosis (AGEP) induced by terbinafine with involuntary positive reintroduction. Eur J Dermatol. 2005; 15: 116.
9. Amouri M, El Euch D, El Ouni B, et al. Acute generalized exanthematous pustulosis due to terbinafine. Eur J Dermatol. 2005 Mar-Apr;15(2):116. [PubMed]
10. Todd P, Halpern S, Munro DD. Oral terbinafine and erythema multiforme. Clin Exp Dermatol. 1995 May;20(3):247-8. [PubMed]
11. Rzany B, Mockenhaupt M, Gehring W, et al. Stevens-Johnson syndrome after terbinafine therapy. J Am Acad Dermatol. 1994 Mar;30(3):509. [PubMed]
12. Roujeau JC. Clinical heterogeneity of drug hypersensitivity. Toxicology. 2005 Apr 15;209(2):123-9. [PubMed]
13. Kardaun SH, Jonkman MF. Dexamethasone Pulse Therapy for Stevens-Johnson Syndrome/Toxic Epidermal Necrolysis. Acta Derm Venereol. 2007;87(2):144-8. [PubMed]
© 2009 Dermatology Online Journal

