Penile pyoderma gangrenosum
Published Web Location
https://doi.org/10.5070/D36v51g77tMain Content
Penile pyoderma gangrenosum
Christy Badgwell MD, and Ted Rosen MD
Dermatology Online Journal 12 (2): 8
Baylor College of Medicine and Michael E. DeBakey Veterans Affairs Medical Center, Houston, Texas. vampireted@aol.comAbstract
We present a case of penile pyoderma gangrenosum (PG) that responded dramatically to an 8-week course of prednisone and has not recurred over a 6-month period. Although quite uncommon, penile PG should be a diagnostic consideration in any patient with non-healing ulcerative lesions of the penis. A correct diagnosis in this situation precludes unnecessary or harmful therapeutic interventions and leads to proper management.
Introduction
Pyoderma gangrenosum (PG) is a rare cutaneous disorder of unknown etiology classically characterized by an ulcerated lesion with an irregular, raised, undermined, purple border surrounding an edematous necrotic base [1]. Lesions are most commonly located on the lower extremities [2]. We report a case of penile PG as support that genital skin involvement is a possible, albeit rare, location for this condition. The diagnosis of PG is one of exclusion, with penile lesions having a substantial differential diagnosis. PG should be suspected in any patient with ulcerative penile lesion(s) who fails to respond to adequate antibacterial or antiviral therapy and who has a negative or non-confirmatory diagnostic evaluation for infectious or other inflammatory conditions.
Clinical synopsis
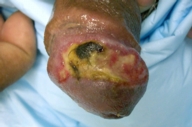
A 54 year-old man presented with a severely painful penile ulcer that had developed over about 1 month. Review of systems was noncontributory. The patient denied genital trauma and had not been sexually active during the preceding 1 year. Medical history disclosed glipizide-controlled type-II diabetes and hyperlipidemia controlled by lovastatin; both medications had been long-standing. Physical examination disclosed a deep, 3 × 1 cm ulcer on the antero-lateral glans penis extending nearly to the corona (Fig. 1). The border was irregular and included areas of peripheral extension elsewhere on the glans. Areas of necrosis were apparent. There was neither inguinal nor femoral adenopathy. Extensive evaluation for sexually transmitted disease (STD) was negative, including RPR, darkfield examination, herpes culture and specific HSV serology, chancroid culture, fungal cultures, and aerobic and anaerobic bacterial cultures. An exhaustive biochemistry panel was normal. A stool specimen was negative for occult blood; the patient refused colonoscopy because of a lack of symptoms referable to the gastrointestinal tract. Additional medical evaluation failed to reveal any evidence for inflammatory bowel disease, hematological abnormality, hepatic or collagen vascular disease or human immunodeficiency virus (HIV) infection.
 |  |
| Figure 1 | Figure 2 |
|---|---|
| Figure 1. Irregularly-bordered 3 × 1 cm ulcer on antero-lateral penis extending to the corona | |
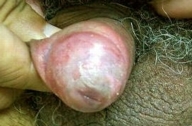
| Figure 2. Penis after prednisone treatment | |
While under the care of primary care physicians and urologists, the patient failed to improve following antibacterial and antiviral therapy (including adequate dosages of the following: ciprofloxacin, amoxicillin-clavulanate, doxycycline, vancomycin, and valacyclovir. Two skin biopsies revealed a nonspecific picture, severe acute and mild chronic inflammation (with a predominance of polymorphonuclear leukocytes within the dermal infiltrate). Special stains for various microbes were negative. The clinical morphology coupled with disabling pain, various therapeutic failures, a negative work up for STD, and a compatible histology all suggested the diagnosis of PG. The patient was placed on an 8-week tapering dose of prednisone (60 mg to start) with complete resolution of the lesion (Fig. 2). There remained only mild residual post-inflammatory dyschromia and superficial scarring. The patient remains disease free after 6 months.
Discussion
PG is an uncommon skin disorder characterized by ulcerative lesions that most often affect the lower extremities but may involve the face, neck, scrotum, or penis [2, 3]. The annual incidence of PG in the United States is approximately 1 case per 100,000 people [3], with patients typically being in the 25-54 year age range [4]. Lesions of PG may begin as a pustule, nodule, or inflammatory bullae that develop into large necrotic, ulcerative plaques [4]. PG ulcers are classically painful and aseptic, although superinfection may eventually occur [5].
The etiology of PG is poorly understood, but approximately 50 percent of PG patients have an associated systemic disorder, with inflammatory bowel disease being the most common [3]. Other diseases associated with PG include rheumatoid arthritis, monoclonal gammopathy (chiefly IgA), polycythemia rubra vera, hematologic malignancies, chronic active hepatitis, HIV infection, systemic lupus erythematosus, Takayasu's arteritis, and Wegener's granulomatosis [4]. Given that the cutaneous lesions of PG may be the presenting manifestation of an underlying disease, a complete work up for systemic disease and regular patient follow up are mandatory.
Because there are no specific diagnostic criteria either by microscopic or laboratory examination, the diagnosis of PG is one of exclusion, resting primarily on clinical features. The histopathology of PG is not diagnostic; PG lesions may demonstrate massive neutrophilic infiltration, edema, engorgement and thrombosis of small- and medium-sized vessels, necrosis, and hemorrhage [2]. Although extensive granulomatous inflammation may be evident rarely histologically, a sterile pyodermatous reaction is more common in PG [2, 4]. Although skin biopsy is not diagnostic, it is imperative in order to exclude malignancy and other more common causes of ulcerative lesions [6]. The differential diagnosis of penile ulcerative lesions includes STD, calciphylaxis, multi-system disease (e.g., Behcet's), necrotizing faciitis, cutaneous metastatic Crohn's disease, deep fungal infection, pemphigus vegetans, Fournier's gangrene, neoplastic conditions, erosive lichen planus, trauma, and factitious damage [3, 4, 5, 7, 8].
Early recognition is critical in order to avoid unnecessary or potentially harmful interventions. For example, surgical debridement is therapeutic in cutaneous metastatic Crohn's disease but may lead to increased tissue loss and disease progression in PG [5]. Similarly, broad-spectrum antibiotics and aggressive surgical therapy are indicated in Fournier's gangrene, but such interventions are not recommended in PG [9]. The extension of lesions in response to trauma or surgical debridement, termed pathergy, is a hallmark of PG [6].
Penile PG is rare, with less than a dozen reported cases to date [1, 2, 3, 4, 6, 8, 9, 10, 11, 12, 13]. The scrotum has been affected in close to half of the reported cases, and there has been one case with associated perianal involvement [9]. Two reported cases of penile PG were associated with systemic chronic lymphocytic lymphoma [11] and ulcerative colitis respectively [9].
Therapeutic options for penile PG include topical or systemic corticosteroids, the mainstays of treatment for PG in general. Additionally, other agents have been used in combination with steroids or as monotherapy. In 1998, Farrell et al. reported the successful use of combination therapy in two patients with penile PG. One patient was treated with prednisolone 80 mg daily, minocycline 100 mg twice daily, and thalidomide 100 mg daily for almost 7 weeks followed by minocycline as monotherapy [13]. The patient later underwent surgery to restore some length to the penis, and prednisolone 40 mg daily and thalidomide 100 mg twice weekly were added to the regimen during the perioperative period to prevent pathergy at the site of surgery. There was no evidence of PG recurrence after 10 months. The second patient was treated with prednisolone 15 mg daily and minocycline 100 mg twice daily for almost 9 months, later experiencing one relapse that was successfully managed by re-introducing minocycline.
Additionally, the beneficial use of oral prednisolone 80 mg per day and sulfasalazine 4 g per day in a case of granulomatous penile PG has been reported [2]. Per report, the medications were gradually discontinued over 4 weeks, and the patient remained disease free after 2 years. Lee et al. later treated a case of penile PG with high-dose prednisone and cyclosporine and reported lesional healing in 8 weeks [6]. Furthermore, topical tacrolimus is reportedly efficacious in clearing penile PG lesions [8]. Although localized infection is a risk with tacrolimus application, this did not occur in the reported patient.
Therapeutic circumcision may be considered in certain cases of PG, as a dysfunctional foreskin may promote extension on the glans and shaft, and PG is known to manifest the Koebner phenomenon [1, 8].
The course of PG commonly parallels that of associated ulcerative colitis (UC) such that surgical or medical treatment of underlying inflammatory bowel disease may assist in the healing of PG lesions. The resolution of UC-associated penile, scrotal, and perineal PG has been reported after a patient's UC was treated via therapeutic colectomy [1].
Conclusion
We present a case of penile PG as a reminder that, although PG of the penis is rare, a progressive, non-healing painful penile lesion with negative laboratory and histopathological evaluations should prompt the consideration of this entity. Because PG lesions may be the presenting manifestation of a primary systemic disorder, a complete work up for underlying disease and regular patient followup are necessary. First-line therapy in most cases of PG involves topical or systemic corticosteroids. Systemic steroids proved to be rapidly efficacious in the present case.
References
1. Sanusi ID, Gonzalez E, Venable DD. Pyoderma gangrenosum of penile and scrotal skin. J Urol 1982 Mar;127(3):547-9. PubMed2. Park HJ, Kim YC, Cinn YW, Yoon TY. Granulomatous pyoderma gangrenosum: two unusual cases showing necrotizing granulomatous inflammation. Clin Dermatol 2000 Nov;25(8):617-20. PubMed
3. Gonzalgo ML, de Lacerda DA, De Marzo AM, Chan DY. Persistent purulent drainage from the glans penis: atypical presentation of pyoderma gangrenosum. J Urol 2003 May;169(5):1793-4. PubMed
4. Gungor E, Karakayali G, Alli N, Artuz F, Lenk N. Penile pyoderma gangrenosum. J Eur Acad Dermatol Venereol 1999 Jan;12(1):59-62. PubMed
5. Sams HH, Kiripolsky MG, Boyd AS, King LE Jr. Crohn's disease of the penis masquerading as pyoderma gangrenosum: a case report and review of the literature. Cutis 2003 Dec;72(6):432-7. PubMed
6. Lee DK, Hinshaw M, Cripps D, Jarrard DF. Pyoderma gangrenosum of the penis. J Urol 2003 Jul;170(1):185-6. PubMed
7. Aviles-Izquierdo JA, Suarez-Fernandez R, Lazaro-Ochaita P, Longo-Imedio I. Metastatic Crohn's disease mimicking genital pyoderma gangrenosum in an HIV patient. Acta Derm Venereol 2005;85(1):60-2. PubMed
8. Lally A, Hollowood K, Bunker CB, Turner R. Penile pyoderma gangrenosum treated with topical tacrolimus. Arch Dermatol 2005 Sep;141(9):1175-6. PubMed
9. Baskin LS, Dixon C, Stoller ML, Carroll PR. Pyoderma gangrenosum presenting as Fournier's gangrene. J Urol 1990 Oct;144(4):984-6. PubMed
10. Soto LD. Diaminodiphenylsulfone and steroids in the treatment of pyoderma gangrenosum. Int J Dermatol 1970 Oct-Dec;9(4):293-300. PubMed
11. Harto A, Gutierrez Sanz-Gadea C, Vives R, Romero Maroto J, Ledo A. [Pyoderma gangrenosum of the penis]. Actas Urol Esp. 1985 May-Jun;9(3):263-6. Spanish. PubMed
12. Herrera Sanchez M, Rojo Sanchez S, del Cerro Heredero M, Rueda Gomez-Calcerrada M, Alvarez Vieitiez A, Suarez Fernandez R, Sanchez Yus E. Pyoderma gangrenosum of penile skin. Int J Dermatol 1997 Aug;36 (8):638-9. PubMed
13. Farrell AM, Black MM, Bracka A, Bunker CB. Pyoderma gangrenosum of the penis. Br J Dermatol 1998 Feb;138 (2):337-40. PubMed
© 2006 Dermatology Online Journal

