Treatment of limited extent extramammary Paget's Disease with 5 percent imiquimod cream
Published Web Location
https://doi.org/10.5070/D36cd2f48fMain Content
Treatment of limited extent extramammary Paget's Disease with 5 percent imiquimod cream
Christy Badgwell MD and Ted Rosen MD
Dermatology Online Journal 12 (1): 22
Department of Dermatology, Baylor College of Medicine, Houston, Texas. vampireted@aol.comAbstract
Extramammary Paget's disease (EMPD) is a rare neoplastic condition of apocrine gland-bearing skin, which may be associated with internal malignancy. Although surgical excision is the generally accepted standard of care for EMPD, treatment with topical imiquimod 5 percent cream has reportedly been efficacious in clearing lesions. We report the case of a 78-year-old woman with biopsy-proven EMPD of the thigh treated successfully with imiquimod application thrice weekly for 16 weeks.
Introduction
Although rare, extramammary Paget's disease (EMPD) is a serious condition because underlying internal malignancy may accompany superficial cutaneous lesions [1]. Patients commonly present with pruritic patches or plaques with a non-specific clinical appearance, thus leading to a significant delay between initial presentation and diagnosis [2]. Surgical excision, the generally accepted standard treatment for EMPD, is associated with a relatively high recurrence rate [3]. For non-invasive, well-defined, unicentric EMPD or for patients refusing surgery, localized treatment with topical imiquimod 5 percent cream may be appropriate. We report a case of EMPD of the thigh successfully treated with imiquimod as evidence of the possible utility of this agent in the management of EMPD.
Clinical synopsis
A 78 year-old Caucasian woman presented with a three month history of a pruritic eruption on the right upper, inner thigh. She was in otherwise good health and review of systems was entirely negative. Physical examination revealed an approximately 6.5 cm by 5.25 cm erythematous plaque on the medial thigh with a small satellite lesion of similar morphology immediately superior (Fig. 1). A 3-mm punch biopsy revealed histological and immunohistochemical features diagnostic of EMPD, including an intra-epidermal proliferation of large, pleomorphic, mucicarmine and carcinoembryonic antigen (CEA) positive-staining, pale cells. An extensive evaluation for underlying genitourinary or gastrointestinal neoplasia was negative. The patient did not wish to have surgical intervention, but she consented to topical therapy with imiquimod 5 percent.
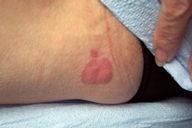 |  |
| Figure 1 | Figure 2 |
|---|---|
| Figure 1. Lesions at initial presentation. | |
| Figure 2. After four weeks of thrice weekly imiquimod therapy. | |
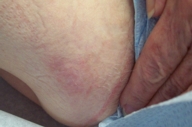
Imiquimod was applied 3-times weekly to both affected skin and to a 5-cm surrounding area. After 4 weeks, the lesion was only slightly more erythematous, and therapy was continued (Fig. 2). Additional treatment for 12 weeks led to increasing erosion; therapy was discontinued after 16 weeks because imiquimod application led to prominent erosion (Figs. 3 and 4). Therapy was well tolerated without significant pain. Bland emollient ointment was applied after treatment was discontinued; although the site re-epithelialized in 2 weeks, obvious erythema remained (Fig. 5). Without additional intervention, the site was nearly normal appearing 6 months later (Fig. 6). Three punch biopsies were performed, and all were negative for residual tumor. No recurrence has been noted during an additional 9-months observation.
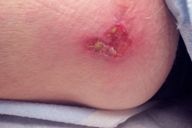 | 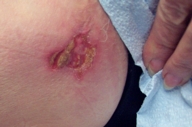 |
| Figure 3 | Figure 4 |
|---|---|
| Figure 3. After 12 weeks of thrice weekly imiquimod therapy. | |
| Figure 4. After 16 weeks of thrice weekly imiquimod therapy. | |
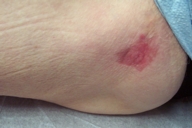 |  |
| Figure 5 | Figure 6 |
|---|---|
| Figure 5. Two weeks after imiquimod discontinuation. | |
| Figure 6. Six months after imiquimod discontinuation. | |
Discussion
EMPD is an uncommon neoplastic condition of apocrine gland-bearing skin that is thought to originate either from the intraepidermal cells of apocrine gland ducts or from pluripotent keratinocyte stem cells [1]. Lesions of EMPD typically involve the vulvar, perianal, perineal, scrotal, and penile regions [2]. More rarely affected areas include the thighs, buttocks, axilla, eyelids, and external ear canal. If EMPD presents in areas relatively free of apocrine glands, it is termed ectopic EMPD [4]. EMPD of the external genitalia may be associated with bladder, urethra, or prostrate cancer; and EMPD of the perianal skin is often related to colorectal neoplasia [1]. In light of these associations, a thorough investigation for an underlying carcinoma should accompany every confirmed diagnosis of EMPD [4].
EMPD generally presents between the ages of 50 and 80, with Caucasian women being the most likely demographic affected. The most common presenting complaint is pruritus [1], but patients may experience burning, irritation, pain, tenderness, bleeding, and swelling [4]. Clinically, lesions are well-demarcated, erythematous or leucoplakic plaques with an eczematous appearance [2, 4]. The nonspecific clinical appearance of EMPD often results in an extended period between the onset of symptoms and diagnosis. The morphology of long standing lesions may be altered by excoriation, superimposed infection or iatrogenic intervention [1].
EMPD, like Paget's disease, is diagnosed via histological demonstration of infiltrating intraepithelial neoplastic cells showing glandular differentiation [5]. The characteristic neoplastic cells are of large size and demonstrate round or oval nuclei with abundant, pale, vacuolated cytoplasm. Immunohistochemical staining carries diagnostic value, as cells often stain positively for alcian blue, periodic acid-Schiff, mucicarmine, cytokeratin 7, and CEA [6]. Simple skin scrapings from EMPD may demonstrate acinar groups consistent with glandular differentiation and malignant cells with both vacuolated cytoplasm and eccentric nuclei [1]. However, such skin samples are be variably cellular and often show a background of keratinous debris that may lead to diagnostic confusion [7]. Therefore, a biopsy of suspicious lesions is more appropriate.
Although surgical excision carries a high recurrence rate, it remains the standard therapy for EMPD [3]. Recurrence is associated with the propensity of EMPD lesions to have irregular margins, mutlicentricity, and involvement of apparently normal skin [8, 9]. Localized treatment modalities such as conventional scalpel surgery, Mohs microsurgery, radiation, topical chemotherapy, photodynamic therapy, and carbon dioxide laser ablation may be appropriate for non-invasive, well-defined, unicentric EMPD [2]. For patients with associated internal malignancy, surgery in addition to chemotherapy and radiation may be indicated [3, 10].
In recent years, treatment of localized EMPD lesions with imiquimod 5 percent cream has been reportedly successful, as in the present case. Imiquimod is an imidazoquinoline amine with immune response modifying effects and potent anti-viral and anti-tumor activity in animal models [11]. Imiquimod is currently FDA approved for topical treatment of external genital warts, actinic keratosis, and superficial basal cell cancer [12]. Common adverse events associated with imiquimod application include local irritation, erythema, superficial erosion to ulceration with eschar formation, pruritus, and pain. More generalized complaints of fever, flu-like symptoms, headache, and myalgia are also possible.
Although the exact mechanism of action is not known, imiquimod has been found to stimulate the innate immune response through induction, synthesis, and release of cytokines such as interferon-α (IFN-α), interleukin-6 (IL-6), and tumor necrosis factor-α (TNF-α) in mice [13, 14]. Additionally, imiquimod is thought to stimulate the cellular arm of acquired immunity via induction of IFN-α, IFN-γ, and IL-12.[11] Increased IFN-α and TNF-α concentrations have been found at the application site after topical administration of 1 percent or 5 percent topical imiquimod onto the skin of hairless mice [15]. Furthermore, imiquimod may involve an activation of the immune response via modulation of Langerhans' cell function, specifically enhancing viral and tumor antigen presentation to naïve T lymphocytes by inducing migration of Langerhans' cells to regional lymph nodes [11].
In 2002 Zampgna et al. reported the successful use of imiquimod to treat a patient with limited cutaneous EMPD of the scrotum and another patient with perineal and crural EMPD [3]. The patient with scrotal EMPD was treated with imiquimod 5 percent cream every other day for a total of 16 weeks after initial daily treatment resulted in the development of local skin breakdown and systemic flu-like symptoms. The lesions cleared, and a post-treatment biopsy was negative for neoplastic cells. Nearly one year later, the patient was reportedly free of signs or symptoms of EMPD. The patient with perineal and crural EMPD was treated with imiquimod every other day for 7.5 weeks with resolution of lesions. Four months after treatment, five biopsies from this patient were histologically free of disease.
Qian et al reported their experience with imiquimod use in a patient with persistent EMPD of the ventral penis despite multiple treatments with aminolevulinic acid-photodynamic therapy [16]. After 6 weeks of thrice weekly application, the area was clear of lesions, and a repeat biopsy showed complete histologic resolution. Per report, the area was clinically free of disease 14 months after treatment.
Successful treatment of vulvar EMPD with 5 percent imiquimod cream was also reported by Wang and co-workers [17]. A patient with vulvar EMPD was initially treated with topical corticosteroids and pimicrolimus 1 percent cream without improvement. She was then treated with imiquimod for a total of 6 weeks, with 6 days of daily application and 5 weeks of twice or thrice weekly application. Two weeks after therapy, there was no clinical evidence of EMPD.
In 2003 Berman et al. reported a case of recurrent crural and scrotal EMPD treated with imiquimod 5 percent cream [18]. The patient was originally treated with Mohs surgery, but he developed a recurrence of lesions 4 years later. Imiquimod was applied daily for 6 weeks, with regression of lesions after 4 weeks of therapy. Skin biopsies at 4 and 6 months after treatment completion showed no cellular atypia.
Conclusion
Although there is not extensive experience with the use of topical imiquimod 5 percent in the treatment of EMPD, the reported cases of successful use of this agent in limited extent EMPD are promising. Larger scope, randomized controlled trials are needed to determine the true safety and efficacy of imiquimod in comparison to the other therapy modalities currently used to manage EMPD. To the best of our knowledge, the treatment of EMPD of the thigh with imiquimod is not reported elsewhere in the literature, and we report our case as further evidence of the potential role imiquimod may play in this disorder.
References
1. Lloyd J, Flanagan AM. Mammary and extramammary Paget's disease. Mammary and extramammary Paget's disease. J Clin Pathol 2000 Oct;53(10):742-9. PubMed2. Zollo JD, Zeitouni NC. The Roswell Park Cancer Institute experience with extramammary Paget's disease. Br J Dermatol 2000 Jan;142(1):59-65. PubMed
3. Zampogna JC, Flowers FP, Roth WI, Hassenein AM. Treatment of primary limited cutaneous extramammary Paget's disease with imiquimod monotherapy: two case reports. J Am Acad Dermatol 2002 Oct;47(4 Suppl):S229-35. PubMed
4. Shepherd V, Davidson EJ, Davies-Humphreys J. Extramammary Paget's disease. BJOG. 2005 Mar;112(3):273-9. PubMed
5. Jones RE Jr, Austin C, Ackerman AB. Extramammary Paget's disease, A critical reexamination. Am J Dermatopathol 1979 Summer;1(2):101-32. PubMed
6. Battles OE, Page DL, Johnson JE. Cytokeratins, CEA, and mucin in the diagnosis and characterization of extramammary Paget's disease. Am J Clin Pathol 1997 Jul;108(1):6-12. PubMed
7. Samarasinghe D, Frost F, Sterrett G, Whitaker D, Ingram D, Sheiner H. Cytological diagnosis of Paget's disease of the nipple by scrape smears: a report of five cases. Diagn Cytopathol 1993;9(3):291-5. PubMed
8. Coldiron BM, Goldsmith BA, Robinson JK. Surgical treatment of extramammary Paget's disease, A report of six cases and reexamination of Mohs micrographic surgery compared with conventional surgical excision. Cancer 1991 Feb 15;67(4):933-8. PubMed
9. Gunn RA, Gallager HS, Vulvar Paget's disease: a topographic study. Cancer. 1980 Aug 1;46(3):590-4. PubMed
10. Voigt H, Bassermann R, Nathrath W, Cytoreductive combination chemotherapy for regionally advanced unresectable extramammary Paget carcinoma. Cancer. 1992 Aug 1;70(3):704-8. PubMed
11. Sauder DN, Immunomodulatory and pharmacologic properties of imiquimod. J Am Acad Dermatol. 2000 Jul;43(1 Pt 2):S6-11. PubMed
12. Aldara® information website: www.3m.com/us/healthcare/pharma/aldara/index.jhtml, accessed October 22, 2005.
13. Dahl MV. Imiquimod: an immune response modifier. J Am Acad Dermatol. 2000 Jul;43(1 Pt 2):S1-5. PubMed
14. Reiter MJ, Testerman TL, Miller RL, Weeks CE, Tomai MA. Cytokine induction in mice by immunomodulator imiquimod. J Leukoc Biol. 1994 Feb;55(2):234-40. PubMed
15. Imberston LM, Beaurline JM, Couture AM, Gibson SJ, Smith RM, Miller RL, Reiter MJ, Wagner TL, Tomai MA. Cytokine induction in hairless mouse and rat skin after topical application of the immune response modifiers imiquimod and S-28463. J Invest Dermatol 1998 May;110(5):734-9. PubMed
16. Qian Z, Zeitoun NC, Shieh S, Helm T, Oseroff AR. Successful treatment of extramammary Paget's disease with imiquimod. J Drugs Dermatol. 2003 Jan;2(1):73-6. PubMed
17. Wang LC, Blanchard A, Judge DE, Lorincz AA, Medenica MM, Busbey S. Successful treatment of extramammary Paget's disease of the vulva with topical 5% imiquimod cream. J Am Acad Dermatol. J Am Acad Dermatol. 2003 Oct;49(4):769-72. PubMed
18. Berman B, Spencer J, Villa A, Poochareon V, Elgart G. Successful treatment of extramammary Paget's disease of the scrotum with imiquimod 5% cream. Clin Exp Dermatol 2003 Nov;28 Suppl 1:36-8. PubMed
© 2006 Dermatology Online Journal

