Metastatic atypical fibroxanthoma
Published Web Location
https://doi.org/10.5070/D366g2q473Main Content
Metastatic atypical fibroxanthoma
Elizabeth K Satter MD MPH
Dermatology Online Journal 18 (9): 3
Naval Medical Center, San Diego, CaliforniaAbstract
Atypical fibroxanthoma (AFX) is an undifferentiated pleomorphic sarcoma that typically has low-grade malignant potential. Although recurrences do occur, only approximately 25 cases of metastasizing AFX have been reported. Herein a patient with an AFX that metastasized and eventuated in death is described. Although AFX remains a diagnosis of exclusion, through this pedagogic case, the clinical, immunohistochemical, and genetic features that can assist in diagnosis are discussed.
Introduction
Atypical fibroxanthoma (AFX) was first described by Helwig as a low-grade sarcoma with a favorable prognosis [1]. However, since inception, it has been the source of controversy. Despite being markedly pleomorphic, metastases occur in less than 1 percent of cases, with the majority preceded by tumor recurrence [1, 2, 3]. Moreover, AFX differs from other soft tissue sarcomas regarding its propensity for lymph node metastases verses hematogenous dissemination. Upon review of the 25 reported cases of metastatic AFX and the present case, metastases are most common to the lymph nodes (58% [15/26]), followed by visceral organs (35% [9/26]), the parotid glands (19% [5/26]), and local cutaneous sites (12% [3/26]), with some patients having metastases to multiple sites [2, 4-7]. The recurrence rate varies from 0-21 percent [2, 3, 6, 8, 9]. However, this rate is likely confounded by how recurrences are defined [8, 9, 10]. Mirza and Weedon examined 89 AFXs and found none recurred or metastasized. They postulate that previously reported recurrent tumors may simply represent residual disease related to inadequate surgical margins [10]. Ang et al also showed no recurrence in 59 AFXs treated by Mohs micrographic surgery, but 2 recurrences in 23 cases treated with wide excision [8]. The largest study to date, showed a recurrence rate of 6 percent (9/140) with 66.6 percent of cases having positive surgical margins, which endorses Mirza’s and Weedon’s theory [3, 10]. Another area of contention pertains to establishing which of the histologic features best characterize AFX versus undifferentiated high-grade pleomorphic sarcoma (UHPS), formerly designated malignant fibrous histiocytoma (MFH). New et al argue that some metastatic AFXs should be evaluated with trepidation, because tumor depth is not reported and subcutaneous involvement favors UHPS [4]. However, Mirza and Weedon found that 12.4 percent of the 89 cases they evaluated had subcutaneous involvement, yet none metastasized [10]. Luzar and Calonje showed even a larger percentage of AFXs with subcutaneous extension, with 42.4 percent of 66 AFXs examined extending into the subcutis. However, they emphasized the extension occurred in an expansile fashion and the tumor remained well-demarcated [11]. Lastly, Helwig and May examined 8 metastatic AFXs and failed to find sufficient evidence to support a direct relationship between depth of invasion and metastases [2]. They asserted that vacular invasion was the most helpful feature to portend metastases [2]; yet others refute this conclusion, stating that tumors with lymphovascular invasion should not even be diagnosed as AFX and are best classified as UHPS [11]. Herein, a patient with metastatic AFX primarily restricted to the dermis that eventuated in death is described. Although AFX remains a diagnosis of exclusion, through this pedagogic case, the clinical, immunohistochemical, and genetic features that can assist in diagnosis of are discussed.
Case report
 |  |
| Figure 1 | Figure 2 |
|---|---|
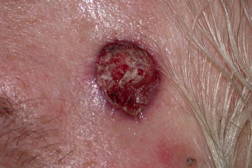
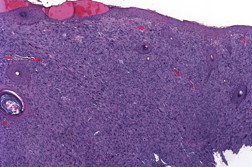
| Figure 1. Tumor at presentation. Figure 2. Pleomorphic spindle cell proliferation with multiple atypical mitoses that replaces the dermis (H&E x40). | |
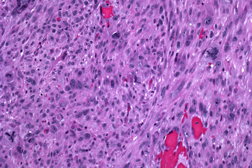
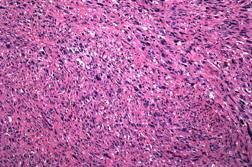
A 63-year-old man presented with a 5-month history of a progressively enlarging 3.2 x 2.0 cm ulcerated nodule on his left temple (Figure 1). Shave biopsy revealed diffuse replacement of the dermis by a proliferation of pleomorphic polyhedral and spindle cells containing multiple mitoses, many of which were atypical (Figure 2 and 3). The tumor exhibited strong diffuse staining with vimentin and CD10, and weak focal staining with CD68 and SMA. It failed to react with pancytokeratin, high molecular weight cytokeratins (34βE12), S100, HMB45, Desmin, CD34, CD99, and LN-2 (CD74). The tumor was excised with clear margins utilizing Mohs micrographic surgery. Because the tumor extended to the base of the original biopsy, the debulking layer was sent for permanent sections to determine the depth of invasion. The tumor was primarily centered in the dermis, but focally invaded the superficial subcutis in an expansile fashion. There was no evidence of vascular invasion or tumor necrosis. Initially the patient did well, but 31 months later, he presented with cough, fever, and a loculated pleural effusion that failed to respond to antibiotics. Five months later, a lung biopsy showed a pleomorphic spindle cell proliferation with IHC staining similar to the original biopsy (Figure 4). Because of multiple co-morbidities, he was discharged under the auspices of hospice and died 2 months later.
 |  |
| Figure 3 | Figure 4 |
|---|---|
| Figure 3. Closer view of the pleomorphic spindle cells and atypical mitoses (H&E x200). Figure 4. Pleomorphic spindle cells in lung biopsy (H&E x100). | |
Discussion
A histological diagnosis of AFX remains one of exclusion, with spindle cell squamous cell carcinoma, spindle cell melanoma, leiomyosarcoma, and UHPS the primary tumors in the differential diagnosis. There are several immunohistochemical (IHC) stains that can help discriminate AFX from other superficial pleomorphic spindle cell malignancies. Nonetheless, it is difficult to differentiate AFX from UHPS based solely upon IHC staining. Both tumors strongly express vimentin and variably stain with CD68 and SMA. However, these stains are nonspecific and fail to point to a particular cell lineage. CD10 and procollagen I are newer IHC stains purported as useful adjunctive markers in helping differentiate AFX from other superficial spindle cell tumors, but they also stain UHPS [12-16]. CD99 was initially touted as useful in discriminating between AFX and UHPS with one study showing 94 percent (16/17) of AFXs having moderate to strong diffuse staining, whereas only 15 percent (4/26) of UHPS showed similar staining [17]. These findings were not supported by another study which only found 15 percent (3/31) of AFXs showed moderate staining [14]; thereby, the efficacy of this IHC stain is still under investigation. LN-2 (CD74) also shows promise in differentiating the two tumors, with 90 percent (18/20) of UHPS showing moderate to strong expression, as compared to only 10 percent (2/20) of AFXs [18]; more studies are needed to draw any definitive conclusions.
Clinically AFX and UHPS differ in their presentation and predominant location in the tissue. Although a comprehensive review of all the differences and exceptions to the rules are beyond the scope of this article, Table 1 gives a brief comparison of these tumors. Classically, AFX arises as a rapidly growing nodule in elderly Caucasians on sun-damaged skin of the head and neck. It is usually <2 cm and is dermal based, but can focally invade the superficial subcutis in an expansile pattern [1-10]. On the other hand, UHPS are typically >5 cm and occur in the deep soft tissue of the proximal extremities unrelated to actinic damage. They have a recurrence rate of 16-39 percent and 30-44 percent metastasize, with 90 percent of metastases to the lung [19].
Multiple factors have been hypothesized to contribute to their divergent biologic behavior. As mentioned earlier, some authors postulate that the depth of involvement may predispose a tumor to more aggressive behavior, similar to that seen with dermal versus soft tissue leiomyosarcoma [4, 20], but other studies dispute this [2, 10, 11]. Although no consistent cytogenic aberrations have been found in either tumor, some authors propose that tumoral pathogenesis may play a role in the differences in their biological behavior because the majority of AFXs studied exhibited ultraviolet-induced p53 gene mutations, which is not seen in UHPS [21]. Recently H-ras and N-ras mutations been shown in some UHPS, but none of the AFXs. However, only a small number of cases were studied [22].
In the present case, the patient presented with a tumor on sun-damaged skin. The tumor was primarily located in the dermis, with no evidence of vascular invasion or necrosis, and had IHC staining consistent with an AFX. However, it was larger than is typical for an AFX, focally extended to the subcutis, and showed aggressive behavior. Therefore, it is uncertain how most appropriately to classify this tumor. Should the tumor be classified as an aggressive AFX that metastasized or a superficial variant of UHPS?
In conclusion, a diagnosis of AFX must be made based upon the cumulative findings of clinical presentation, histological features, and a panel of IHC stains to exclude other tumors. It is important to report unusual cases of AFX because, although the incidence of metastases is typically low, the potential for aggressive behavior exists and the clinical and histological characteristics of these cases may better help determine the factors that contribute to a poorer prognosis.
References
1. Helwig EB. Atypical fibroxanthoma. In: Seminar Proceedings of 18th Annual Seminar of San Antonio Society of Pathologists, 1961. Tex State J Med. 1963; 59: 664-667.2. Helwig EB, May D. Atypical fibroxanthoma of the skin with metastasis. Cancer. 1986; 57: 368-376. [PubMed]
3. Fretzin DF, Helwig EB. Atypical fibroxanthoma of the skin. A clinicopathologic study of 140 cases. Cancer. 1973;31:1541-1552. [PubMed]
4. New D, Bahrami S, Malone J, Callen JP. Atypical Fibroxanthoma with regional lymph node metastasis. Arch Dermatol. 2010;146(12):1399-1404. [PubMed]
5. Glavin FL, Cornwell ML. Atypical fibroxanthoma of the skin metastatic to the lung. Am J Dermatopathol. 1985;7:57-63. [PubMed]
6. Davis JL, Randle HW, Zalle MJ, Roenigk RK, Broadland DG. A comparison of Moh’s micrographic surgery and wide excision for the treatment of atypical fibroxanthoma. J Dermatol Surg. 1997;23:105-110. [PubMed]
7. Rizzardi C, Angiero F, Melato M. Atypical fibroxanthoma and malignant fibrous histiocytoma of the skin. Anticancer Res. 2003;23(2C):1847-51. [PubMed]
8. Ang GC, Roenigk RK, Otley CC, Philips PK, Weaver AL. More Than 2 Decades of Treating Atypical Fibroxanthoma at Mayo Clinic: What Have We Learned From 91 Patients? Dermatol Surg. 2009;35:765-772. [PubMed]
9. Dettrick A, Strutton G. Atypical fibroxanthoma with perineural or intraneural invasion: report of two cases. J Cutan Pathol. 2006;33:318-22. [PubMed]
10. Mirza B, Weedon D. Atypical fibroxanthoma: a clinicopathological study of 89 cases. Australas J Dermatol. 2005;46:235-8. [PubMed]
11. Luzar B, Calonje E. Morphological and immunohistochemical characteristics of atypical fibroxanthoma with a special emphasis on potential diagnostic pitfalls. J Cutan Pathol. 2010;37:301-309. [PubMed]
12. Weedon D, Williamson R, Mirza B. CD10, a useful marker for atypical fibroxanthomas. Am J Dermatopathol. 2005;27:181. [PubMed]
13. de Feraudy S, Mar N, McCalmont TH.Evaluation of CD10 and procollagen 1 expression in atypical fibroxanthoma and dermatofibroma. Am J Surg Pathol. 2008;32:1111-1122. [PubMed]
14. Kanner WA, Brill LB 2nd, Patterson JW, Wick MR. CD10, p63 and CD99 expression in the differential diagnosis of atypical fibroxanthoma, spindle cell squamous cell carcinoma and desmoplastic melanoma. J Cutan Pathol. 2010;37:744-50. [PubMed]
15. Clarke LE, Frauenhoffer E, Fox E, Neves R, Bruggeman RD, Helm KF. CD10-positive myxofibrosarcomas: a pitfall in the differential diagnosis of atypical fibroxanthoma. J Cutan Pathol. 2010;37:737-743. [PubMed]
16. Jensen K, Wilkinson B, Wines N, Kossard S. Procollagen 1 expression in atypical fibroxanthoma and other tumors. J Cutan Pathol. 2004;31:57-61. [PubMed]
17. Hartel PH, Jackson J, Ducatman BS, Zhang P. CD99 immunoreactivity in atypical fibroxanthoma and pleomorphic malignant fibrous histiocytoma: a useful diagnostic marker. J Cutan Pathol. 2006;33(Suppl. 2):24-28. [PubMed]
18. Lazova R, Moynes R, May D, et al. LN-2 (CD74) A marker to distinguish atypical fibroxanthoma from malignant fibrous histiocytoma. Cancer. 1997;79:2115-2124. [PubMed]
19. Fletcher CDM, van den Berg E, Molenaar. Pleomorphic malignant fibrous histiocytoma/Undifferentiated high grade pleomorphic sarcoma. In WHO Classification of tumors. Pathology and genetics of tumors of soft tissue and bone. Eds Fletcher CDM, Unni KK, Mertens F. Lyon: IARC Press; 2002.120-122.
20. Fauth CT, Bruecks AK, Temple W, Arlette JP, DiFrancesco LM. Superficial leiomyosarcoma: a clinicopathologic review and update. J Cutan Pathol. 2010;37:269-276. [PubMed]
21. Dei Tos AP, Maestro R, Doglioni C, et al: Ultraviolet-induced p53 mutations in atypical fibroxanthoma. Am J Pathol. 1994;145:11-17. [PubMed]
22. Sakamoto A, Oda Y, Itakura E, Oshiro Y, Tamiya S, Honda Y, Ishihara A, Iwamoto Y, Tsuneyoshi M. H-, K-, and N-ras gene mutation in atypical fibroxanthoma and malignant fibrous histiocytoma. Hum Pathol. 2001;32:1125-12. [PubMed]
© 2012 Dermatology Online Journal

