A case with widespread cutaneous metastases of unknown primary origin: Grave prognostic finding in cancer
Published Web Location
https://doi.org/10.5070/D35xq3k59jMain Content
A case with widespread cutaneous metastases of unknown primary origin: Grave prognostic finding in cancer
Rafet Koca MD1, Yucel Ustundag MD2, Eksal Kargi MD3, Gamze Numanoglu MD4, H. Cevdet Altinyazar MD1
Dermatology Online Journal 11 (1): 16
Departments of Dermatology1, Gastroenterology2, Reconstructive Surgery3, and Pathology4, Zonguldak Karaelmas University, School of Medicine, Turkey. rafetkoca@karaelmas.edu.tr
Abstract
We present a 52-year-old man with widespread cutaneous metastases (CMs) of unknown primary origin. Although we performed many of the investigations, we could not find out a primary origin of malignancy. There are no practical algorithms to identify the primary of cutaneous metastatic tumors of unknown origin. An algorithm in cancer patients with CMs seems to be needed to manipulate such cases. We believe that more reports related with this issue must be published to form an algorithm in such cases.
Introduction
Cutaneous metastases (CMs) are a prognostically important diagnosis. CMs are not an uncommon occurrence, and this has been reported in 0.7-9 percent of all patients with cancer [1]. The most common sites of primary malignancy in patients who presented with CMs is breast cancer in women and lung cancer in men [2]. The primary site of metastases usually can be identified, but in some cases the primary remains unknown. Metastatic cancer of unknown origin is present in 5-10 percent of all malignancies [3]. CMs are usually associated with known primary disease. A total of 4.4 percent of all patients with CMs present without an identifiable primary site [4]. We present a case with CMs of unknown-primary origin and briefly discuss the need for an algorithm in investigating the primary in such cases.
Clinical synopsis
A 52-year-old man is referred to the gastroenterology clinic with severe epigastric pain and lumbago for 1 month. His symptoms do not respond to oral analgesic. On physical examination, there is a round, indurated, fixed, right-submandibular mass measuring 3 by 4 CMs. The rest of his physical examination is normal, including ear, nose, and throat regions. His routine blood biochemistry and hemogram are unremarkable (Table 1).
An abdominal ultrasonography delineates multiple liver metastases. Abdominal computed tomography (CT) scan reveals minimal hepatomegaly and multiple masses in the liver and multiple lymphadenopathies in the abdomen. There is no abnormalities on CT examination of the chest and pelvis. The thyroid gland was normal on ultrasonography. The fine-needle aspiration biopsy from the submandibular mass is diagnosed as malignant epithelial tumor without an identifiable primary site. Examination with gastroscopy reveals multiple ulcers on gastric corpus and duodenum. Multiple biopsies are taken from the periphery of corpus ulcers. Histopathologic diagnosis is made as malignant epithelial tumor with the morphological features of unknown primary. Immunohistochemical stains of gastric biopsies with vimetin and pan-cytokeratin are positive; leukocyte common antigen (LCA) stain was negative. The serum levels of tumor markers such as carcino-embryogenic antigen (CEA), α-fetoprotein, CA 15-3, CA 19-9 are normal. The patient is diagnosed as metastatic malignant epithelial tumor of unknown primary origin. His performance status precludes any form of chemotherapy.
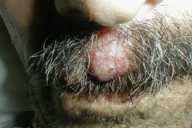
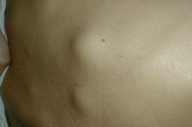
The patient presents 1 month later with nausea, weight loss, and cutaneous nodules that have emerged rapidly. On physical examination, firm, fixed, and painless multiple cutaneous nodules with erythema of overlying skin are distributed on his face and scalp, and firm subcutaneous nodules varying from 0.5-2 cm in size are present on the back.

|
|
| Figure 1 | Figure 2 |
|---|---|
| Figure 1. Cutaneous nodule on the scalp. | |
| Figure 2. Cutaneous nodule on the face. | |
|
| Figure 3 |
|---|
| Figure 3. Subcutaneous nodules on the back. |
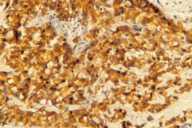
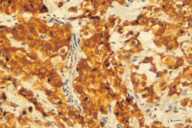
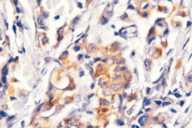
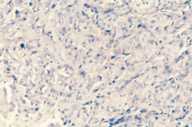
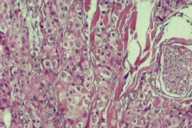
Additional radiological investigations show multiple metastases in both lungs. There had been no lesions in both lungs previously on radiological examinations. Histological examination of the excised nodule from the scalp reveals nests of tumor cells with eosinophilic cytoplasm, large nuclei, and evident nucleoli present among collagen fibrils in the dermis. Numerous mitotic figures are present.
|
| Figure 4 |
|---|
| Nest of tumor cells occupied among collagen fibrils in the dermis. (H&E; original magnification, 10x20). |
Immunohistochemical stains with EMA (epithelial membrane antigen, epithelial stain), pancytokeratin (epithelial stain) and vimentin (mesenchymal stain) are positive. HMB-45 (a marker for malignant melanoma), LCA (a marker for lymphoid cells), actin (smooth-muscle marker) and CEA (a marker for colorectal cancer) are also negative (see Table 2).
An obvious primary site of malignancy cannot be identified. The patient is diagnosed as CMs of unknown primary origin. The patient's condition deteriorates progressively, and he dies 2 months after the diagnosis of CMs.
| Tumor markers | Related malignancy |
|---|---|
| Carcino-embryogenic antigen | Gastrointestinal malignancies (colorectal, stomach, hepato-biliary), lung cancer |
| α-fetoprotein | Hepatocellular carcinoma |
| CA 15-3 | Breast cancer |
| CA 19-9 | Pancreas cancer |
Discussion
Cutaneous involvement may occur by three different mechanisms: direct invasion, local metastatic disease, or distant metastasis [4]. It has been thought that the primary tumors likely to metastasize to the skin are those that invade veins, such as kidney and lung carcinomas [5]. The range of clinical presentations extends from localized and nodular to widespread and inflammatory [5]. To our knowledge, there are no practical algorithms to identify the primary of cutaneous metastatic tumors of unknown origin. Although we performed many of the investigations ourselves, we could not find a primary origin of malignancy. CMs commonly occurs in patients with the breast, lung, and kidney cancers [2]. In the present case the lung metastases develop after skin involvement. Thus the primary site is not the lung itself. There is no abnormality in his breast tissue, and the kidneys are normal on radiological investigations. We also scrutinized submandibular mass in suspect with its possible origin as a primary site. In our case, the histological examination of biopsy samples from the submandibular mass indicate a malignant epithelial tumor. Approximately 10-15 percent of of salivary gland tumors occur in the submandibular gland, and, of these, 40-50 percent are malignant. However, metastases to the skin from submandibular gland are very rare [6]. Thus, a submandibular origin in our case is a less likely source of the primary tumor. Oropharyngeal cancers may produce metastases to the face and neck [7], but in this case the oropharyngeal region is free of any pathology. Approximately 95 percent of liver malignancies are metastatic in origin [8]. Our patient has no apparent risk factors for the development of hepatocellular cancer. Therefore, we consider the masses in the liver to be metastatic involvement.
Immunohistochemical staining with CK7, CK20, p63 and CD10 may be the next step in this case. CK7+/CK20+ carcinomas are those from pancreas (cholangiocarcinoma and urothelium) and one-third of gastric carcinomas. The usually CK7+/CK20- tumors include lung, breast, endometrium, ovary, thyroid, salivary gland, and mesothelioma. CK7-/CK20+ tumors are those from large bowel merkel-cell carcinoma and gastric adenocarcinomas. Finally, typically CK7-/CK20- tumors are carcinomas of adrenal cortex, liver, kidney, adrenal gland, prostate, and thymus [9]. CD10, the common acute lymphoblastic leukemia antigen (CALLA), can be used to define malignant cells designated as common acute lymphoblastic leukemia cells. In normal lymphoid ontogeny, CD10 is expressed on pro-B cells and also on mature germinal center B cells. Approximately 75 percent of precursor B-cell acute lymphocytic leukemias express CD10 [10]. The antigen is a neutral endopeptidase and is also found on immature thymocytes and a variety of normal and neoplastic cell types [9]. The p63 gene, a recently identified homologue of the p53 gene, has been reported to be essential in the development of epithelia and is consistently expressed by basal stem cells of stratified epithelium and myoepithelial cells of breast and salivary glands [9, 11].
Immunhistochemical staining usually does not provide further knowledge about primary origin. If immunhistochemical staining indicates an anaplastic epithelial tumor rather than chemotherapy-responsive tumors like lymphoma and germ-cell tumors, these patients have a poor prognosis. Implicit in the concept is that exhaustive multysystem workups are mostly not helpful in such cases, because CMs are generally a terminal finding in cancer patients, indicating approaching death. Nevertheless, an unsophisticated algorithm in cancer patients with CMs is needed to evaluate such cases in order to determine which patients need chemotherapy or further treatment modalities. As far as we know from the literature, there is an insufficient accumulation of knowledge in patients with CMs from unknown primary. We believe that there must be more reports on these patients in order to create an adequate algorithm.
References
1. Spencer PS, Helm TN. Skin metastases in cancer patients. Cutis. 39: 119-21, 1987.2. Schwartz RA. Cutaneous metastatic disease. J Am Acad Dermatol. 33: 161-82, 1995.
3. Greco FA, Hainsworth JD. Cancer of unknown primary site. In: Cancer: Principles and practice of oncology. De-Vita VT, Hellman S, Rosenberg SA, eds. 4th edn. Lippincott, Philadelphia, 1993, p. 2072-92.
4. Lookingbill DP, Spangler N, Sexton FM. Skin involvement as the presenting sign of internal carcinoma. A retrospective study of 7316 cancer patients. J Am Acad Dermatol. 22: 19-26, 1990.
5. Brenner S, Tamir E, Maharshak, Shapira J. Cutaneous manifestations of internal malignancies. Clin Dermatol. 19: 290-7, 2001.
6. KaplanMJ, Johns ME. Malign Neoplasms. In: Otolaryngology-Head and Neck Surgery. Cummings CW, Fredrickson JM, Harker LA, Krause CJ, Schuller DE, eds. 2 edn, Vol. 2. Mosby Year Book, Missouri, 1993, p. 1043-78.
7. Carroll MC, Fleming M, Chitambar CR, Neuburg M. Diagnosis, workup, and prognosis of cutaneous metastases of unknown primary origin. Dermatol Surg. 28: 533-5, 2002.
8. Kew MC. Hepatic tumors and cyts. In: Sleisenger and Fordtran's Gastrointestinal and Liver disease. Feldman M, Scharschmidt BF, Sleisenger MH, eds. 6 edn, Vol. 2. WB Saunders Company, 1998, p. 1364-87.
9. Rosai J. Special techniques in surgical pathology. In: Rosai and Ackerman's Surgical Pathology. Ninth edn., Vol. 1. Mosby, Philadelphia, 2004, p. 37-91.
10. Uherova P, Ross CW, Schnitzer B, Singleton TP, Finn WG. The clinical significance of CD10 antigen expression in diffuse large B-cell lymphoma. Am J Clin Pathol. 115: 582-8, 2001.
11. Ivan D, Hafeez DA, Prieto VG. Expression of p63 in primary cutaneous adnexal neoplasms and adenocarcinoma metastatic to the skin. Mod Pathol. 18: 137-42, 2004.
© 2005 Dermatology Online Journal