An unusual case of adult disseminated cutaneous Langerhans cell histiocytosis
Published Web Location
https://doi.org/10.5070/D357h5m3sdMain Content
An unusual case of adult disseminated cutaneous Langerhans cell histiocytosis
Hamideh Moravvej MD1, Maryam Yousefi MD2, Behrooz Barikbin MD1
Dermatology Online Journal 12 (6): 13
1. Skin research center and department of dermatology, Shohada-e-tajrish hospital, Shahid Beheshti University of Medical Sciences,
Tehran, Iran. bbarikbin@yahoo.com 2. Department of dermatology,Razi hospital, Tehran University of Medical Sciences, Tehran, Iran
Abstract
Langerhans cell histiocytosis (LCH) represents a group of rare histiocytic syndromes characterized by tissue infiltration with dendritic cells. The management of LCH is difficult because these disorders respond inconsistently to immunosuppressive and chemotherapeutic strategies. We describe a refractory and relapsing case of skin and nail limited LCH in a 27-year-old man. He presented with a 7-year history of an erythematous papular eruption of the scalp, ears, face, trunk, axillae, groins, fingernails, feet, and toenails. Diagnosis of LCH was made based on skin histopathology and immunohistochemical staining. Histological studies of biopsy specimens revealed a dense infiltrate of histiocytic mononuclear cells beneath the epidermis; these cells reacted strongly with anti-S-100 antibodies. In addition, CD1a was positive in most of the infiltrating cells. Extensive investigations failed to detect systemic involvement. The patient's cutaneous eruption did not respond to various therapeutic interventions, including phototherapy with oral psoralen with long-wave UV radiation in the A range (PUVA) and cyclosporine. Marked but temporary clinical improvement was achieved with thalidomide, etoposide with systemic steroid, and total body electron beam radiotherapy. Now the patient is on maintenance therapy with thalidomide and is under acceptable control.
Introduction
Langerhans cell histiocytosis (LCH) is a clonal proliferative disease of Langerhans cells that express an immunophenotype positive for S100 and CD1a, and which contain cytoplasmic Birbeck granules. LCH occurs worldwide and most commonly develops in children aged 1-3 years, although disease can develop at any age. An annual incidence of at least 5 per million children has been reported for LCH, with the adult incidence suspected to be less than one-third that of children. LCH is more common in boys, with a male:female ratio of nearly 2:1. In adults, there may be a slight female predominance [1].
Langerhans cell histiocytosis most frequently involves bone, but also involves the skin in 40 percent of cases; in 10 percent of patients it is limited to the skin. Cutaneous findings of skin-limited LCH include scaly papules, vesicles, nodules, tumors with erosion, ulceration, crusting, or purpura [2]. Nail involvement in LCH is rare, but findings include paronychia, nail-fold destruction, onycholysis, and subungual expansion with nail plate loss [3].
Adult LCH is much rarer than infant or childhood LCH; the most commonly involved sites are the skin, lung, and bone [1]. Adult LCH is also more difficult to treat. Disseminated multisystem adult LCH has a more benign chronic course; it has a slowly progressive or undulating course in contrast with the childhood type [1, 2].
We report an interesting case of adult skin and nail limited LCH occurred in a 27 year old man.
Clinical synopsis
A 27-year-old man presented with 7-year history of multiple erythematous papules and nodules on the face, scalp, trunk, axillae, hands, and feet. The lesions were very pruritic.
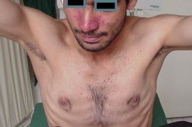
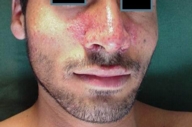
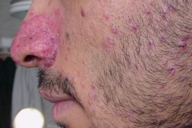
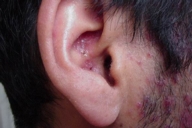
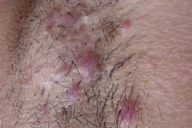
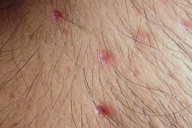
Examination showed symmetrically distributed papules and nodules; some of them were skin colored and some were erythematous and purpuric. The lesions were located on the scalp, forehead, nose and perinasal area, upper lip, chin, cheeks, preauricular areas, ears (conchae and around external auditory meati), retroauricular areas, chest, abdomen, posterior trunk, axillae, and groins (Figs. 1-9). Around the nostrils the papules were confluent. Some of the facial and ear lesions were ulcerated and crusted. Atrophic scars were present at the sites of spontaneously healed lesions (Fig. 10).
 |  |
| Figure 1 | Figure 2 |
|---|---|
| Figure 1. Involvement of the face, chest and axillae by cutaneous LCH Figure 2. Papular lesions on and around the nose and upper lip | |
 |  |
| Figure 3 | Figure 4 |
|---|---|
| Figure 3. Papular lesions on the face | |
| Figure 4. Involvement of the ear | |
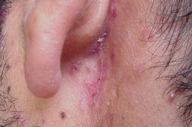 |  |
| Figure 5 | Figure 6 |
|---|---|
| Figure 5. Involvement of the retroauricular area | |
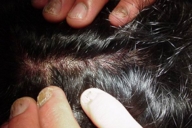
| Figure 6. Involvement of the scalp | |
 |  |
| Figure 7 | Figure 8 |
|---|---|
| Figure 7. Papular lesions on the chest and abdomen | |
| Figure 8. Involvement of the posterior upper trunk | |
 |  |
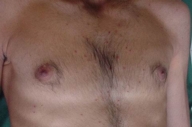
| Figure 9 | Figure 10 |
|---|---|
| Figure 9. Involvement of the axilla | |
| Figure 10. Atrophic scars of the spontaneously healed lesions on the chest | |
 |
| Figure 11 |
|---|
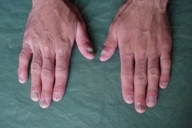
| Figure 11. Involvement of the fingernails |
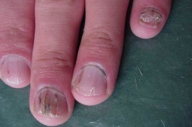
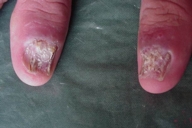
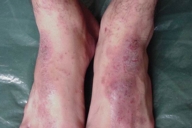
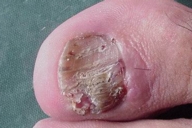
Acral involvement also was present in the form of fingernail involvement of the hands and an eczematous like eruption and nail involvement on the feet (Fig. 11). Nail involvement included brittleness, longitudinal ridging, purpuric lines, V-shaped notches, and pterygium formation (Figs. 11-15).
 |  |
| Figure 12 | Figure 13 |
|---|---|
| Figure 12. Nail involvement in the forms of brittleness, longitudinal ridging, purpuric lines and V-shaped notch | |
| Figure 13. Pterygium formation | |
 |  |
| Figure 14 | Figure 15 |
|---|---|
| Figure 14. Eczema-like lesions on the dorsum of the feet | |
| Figure 15. Toenail involvement | |
The patient denied systemic complaints including fever, significant weight loss, vomiting, diarrhea, polyuria, polydypsia, bone pain, fatigue, dyspnea, and jaundice. On physical examination the head and neck area was normal except the skin lesions on the scalp and face. There was no exophthalmia. The oral cavity, teeth, and gingiva were normal. There was no lymphadenopathy and no organomegally. Chest and abdomen examinations were normal. Neurologic examination including sensory and motor function revealed no abnormality.
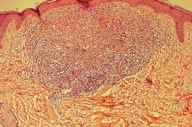
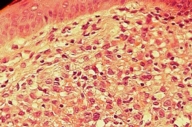
The clinical differential diagnoses included histiocytosis, Darier disease, sarcoidosis, seborrheic dermatitis, and generalized eruptive histiocytoma. Skin biopsies of the neck and nose lesions showed upper and mid-dermal infiltrates composed of large, rounded cells with indented, kidney-shaped nuclei (Figs. 16 and 17).
 |  |
| Figure 16 | Figure 17 |
|---|---|
| Figure 16. Histiocytic infiltration of the upper and mid dermis | |
| Figure 17. Close-up view of fig.16 | |
Immunohistochemistry revealed S-100 protein and CD1a positivity in most of the infiltrating cells. Laboratory tests, PPD skin test, radiologic and ultrasonographic images were all normal. Chest, abdomen and brain CT scans were all normal. A whole body bone scan was normal.
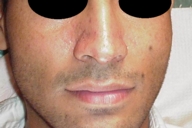
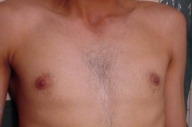
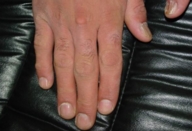
The patient was diagnosed with isolated cutaneous LCH. He received 15 sessions of PUVA therapy with no improvement. He then received thalidomide (100 mg/day for 3 months) with a good response, however, because of occurrence of neuropathy, the drug was stopped and the lesions recurred 1 month later. With the recommendations of oncology department, the patient underwent treatment with etoposide and systemic steroids. He responded with gradual clearing of skin and nail lesions (Figs.18-20).
 |  |
| Figure 18 | Figure 19 |
|---|---|
| Figure 18. Clinical improvement after several sessions of chemotherapy with etoposide plus systemic steroid | |
| Figure 19. Clearance of the chest lesions | |
 |
| Figure 20 |
|---|
| Figure 20. Improvement of fingernail lesions |
The lesions recurred 2 months after termination of treatment. The patient was then treated with total body electron beam radiotherapy and the lesions disappeared again. And again the lesions relapsed 2 months after therapy. Cyclosporine was administered but was completely ineffective. At last, we again started thalidomide with close monitoring for neuropathy and the patient responded well to the drug. At 6 months followup the eruption is under control with maintenance therapy (100 mg twice weekly).
Discussion
LCH is well known in childhood as Abt-Letterer-Siwe disease. The classification of Langerhans-cell histiocytosis and non-Langerhans-cell histiocytosis was established in 1987 by the Histiocyte Society. The group of LCH includes congenital self-healing histiocytosis of the Hashimoto-Pritzker type, Abt-Letterer-Siwe disease, Hand-Schüller-Christian disease, and eosinophilic granuloma. The latter three were formerly called histiocytosis X, where the X indicated the unknown etiology. These have multiple clinical manifestations, and may also involve lymph nodes and bones as a multifocal disease [4].
Initial symptoms occurring in adulthood as in our case are unusual, but this has been previously reported [4, 15, 18, 19, 20, 21]. Recently, Howarth et al. reported 314 cases of various types of LCH, 114 were osseous, 96 were multisystemic, and 104 were single-system non-osseous in origin [17]. Among 104 cases of single-system non-osseous origins, only 14 cases originated in mucocutaneous tissue [17]. Therefore primary cutaneous LCH as in presented case appears to be rare [16]. Chronic relapse of the disease is often seen, and even after some years patients may progress to a multisystem disease [6].
The therapeutic regimen for LCH depends on the localization and extent of the disease. Spontaneous healing is reported as are therapy-resistant cases [7]. Successful treatment with PUVA [8] , thalidomide [9], low dose methotrexate [15] and interferon-α [2] is reported. Intravenous 2-chlorodeoxyadenosine only showed a transient improvement whereas long-term remission was seen after local radiation [10]. In the case of low-risk LCH in children, effective therapy with oral methotrexate and alternate-day prednisolone has been reported [14]. In our case there were no obvious effects of systemic PUVA therapy administered for a period of 2 months.
Thalidomide is an anti-inflammatory, immunomodulatory, and anti-angiogenic compound. These features allow the use of this drug in inflammatory disorders, neoplasms (including multiple myeloma, prostate cancer, renal cell carcinoma, glioma, and colorectal cancer), and in the dermatological manifestations of sarcoidosis and mucocutaneous LCH [23, 24, 25, 26]. The mechanisms that underlay its properties rely on the modulation of inflammatory cytokines such as TNF-α , interferon-γ, IL-10, IL-12, and the nuclear transcription factor NFkB [23, 24]. In the literature there is also evidence that in LCH not only thalidomide but also agents that selectively neutralizes TNF-α activity (etanercept), proved effective in the control of the disease [27].
This case is unusual for involvement limited to skin, the pattern of nail involvement, the age of the patient. It illustrates the relapsing nature of LCH after using several therapies. The response of this patient suggests that thalidomide may be a valuable therapeutic option in disseminated cutaneous LCH, but close monitoring for neuropathy should be performed.
References
1. Warren T Goodman, Terry L Barrett. Disorders of Langerhans cells and macrophages, in textbook of Dermatology edited by Jean L Bolognia, Joseph L Jorizzo, Ronald P Rapini.2003;vol.2:1429-14332. S.E. Chang, G.J. Koh, J.H. Choi, K.J. Sung, K.C. Moon, J.K. Koh.Widespread skin-limited adult Langerhans cell histiocytosis: Long-term follow-up with good response to interferon alpha. Clin Expl Dermatol 2002; 27:135-137. PubMed
3. de Berker, Lever LR, Windebank K. Nail features in Langerhans cell histiocytosis. Br J Dermatol 1994;130:523-7. PubMed
4. Stefanato CM, Andersen WK, Calonje E et al. Langerhans cell histiocytosis in the elderly: a report of three cases. J Am Acad Dermatol 1998; 39: 375 8. PubMed
5. Munn S & Chu AC. Langerhans cell histiocytosis of the skin. Hematol Oncol Clin North Am 1998; 12: 269 86. PubMed
6. Chu AC. The confusing state of the histocytoses. Br J Dermatol 2000; 143: 475 6. PubMed
7. Baldo A, Vitiello A, Argenziano G et al. Pure cutaneous relapsing Langerhans cell histiocytosis in an adult. Eur J Dermatol 1998; 8: 501 2. PubMed
8. Gerlach B, Stein A, Fischer R et al. Langerhanszell-Histiozytose im Alter. Hautarzt 1998; 49: 23 30.
9. Lair G, Marie I, Cailleux N et al. Histiocytose Langerhansienne de l'adulte: localisations cutanéomuqueuses régressives après traitment par thalidomide. Rev Med Interne 1998; 19: 196 8.
10. Conias S, Strutton G, Stephenson G. Adult cutaneous Langerhans-cell histiocytosis. Australas J Dermatol 1998; 39: 106 8. PubMed
11. Rentsch JL, Martin EM, Harrison LC, Wicks IP. Prolonged response of multicentric reticulohistiocytosis to low dose methotrexate. J Rheumatol 1998; 25: 1012 15. PubMed
12. Cash JM, Tyree J, Recht M. Severe multicentric reticulohistiocytosis: disease stabilization achieved with methotrexate and hydroxychloroquine. J Rheumatol 1997; 24: 2250 3. PubMed
13. Liang GC & Granston AS. Complete remission of multicentric reticulohistiocytosis with combination therapy of steroid, cyclophosphamide, and low-dose pulse methotrexate. Arthritis Rheum 1996; 39: 375 8. PubMed
14. Womer RB, Anunciato KR, Chehrenama M. Oral methotrexate and alternate-day prednisone for low-risk Langerhans cell histiocytosis. Med Paediatr Oncol 1995; 20: 70 3. PubMed
15. Steen AE, Steen KH, Bauer R, Bieber T. Successful treatment of cutaneous Langerhans cell histiocytosis with low-dose methotrexate. Br J Dermatol. 2001 Jul;145(1):137-40. PubMed
16. Itoh H, Miyaguni H, Kataoka H, Akiyama Y, Tateyama S, Marutsuka K, Asada Y, Ogata K, Koono M. Primary cutaneous Langerhans cell histiocytosis showing malignant phenotype in an elderly woman: report of a fatal case. J Cutan Pathol. 2001 Aug;28(7):371-8. PubMed
17. Howarth DM, Gilchrist GS, Mullan BP, Wiseman GA, Edmonson JH, Schomberg PJ. Langerhans cell histiocytosis; diagnosis, natural history, management, and outcome. Cancer1999; 85: 2278. PubMed
18. Stockschlaeder M, Sucker C. Adult Langerhans cell histiocytosis. Eur J Haematol. 2006 Mar 17; [Epub ahead of print]. PubMed
19. Walia SS, Gayer K. An unusual presentation of refractory cutaneous Langerhans cell histiocytosis in an adult. J Drugs Dermatol. 2006 Feb;5(2):174-7. PubMed
20. Manfredi M, Corradi D, Vescovi P. Langerhans-cell histiocytosis: a clinical case without bone involvement. J Periodontol. 2005 Jan;76(1):143-7. PubMed
21. Gotz G, Fichter J. Langerhans'-cell histiocytosis in 58 adults. Eur J Med Res. 2004 Nov 29;9(11):510-4. PubMed
22. Sander CS, Kaatz M, Elsner P. Successful treatment of cutaneous langerhans cell histiocytosis with thalidomide. Dermatology. 2004;208(2):149-52. PubMed
23. Franks ME, Macpherson GR, Figg WD. Thalidomide. Lancet 2004;363: 1802 1811. PubMed
24. Moreira AL, Sampaio EP, Zmuidnas A, Frindt P, Smith KA, Kaplan G. Thalidomide exerts its inhibitory action on tumor necrosis factor alpha by enhancing m-RNA degradation. J Exp Med 1993;177: 1675 1680. PubMed
25. Thomas L, Ducros B, Secchi T, Balme B, Moulin G. Successful treatment of adult's Langerhans cell histiocytosis with thalidomide. Report of two cases and literature review. Arch Dermatol 1993;129: 1261 1264. PubMed
26. Kolde G, Schulze P, Sterry W. Mixed response to thalidomide in adults: two cases of multisystem Langerhans' cell histiocytosis. Acta Derm Venereol 2002;82: 384 386. PubMed
27. Henter JI, Karlen J, Calming U, Bernstrand C, Andersson U, Fadeel B. Successful treatment of Langerhans'-cell histiocytosis with etanercept. N Engl J Med 2001;345: 1577 1578. PubMed
© 2006 Dermatology Online Journal

