Management of allergy to heparins in postoperative care: Subcutaneous allergy and intravenous tolerance
Published Web Location
https://doi.org/10.5070/D35368s5x8Main Content
Management of allergy to heparins in postoperative care: Subcutaneous allergy and intravenous tolerance
Cornelia S Seitz1, Eva-B. Brocker2, Axel Trautmann3
Dermatology Online Journal 14 (9): 4
1. Department of Dermatology, Venerology and Allergology, University of Würzburg, Germany. seitz_c@klinik.uni-wuerzburg.de2. Direktorin der Universitäts-Hautklinik, Julius-Maximilians-Universität Würzburg, Head of Department of Dermatology, University of Wuerzburg. broecker_e@klinik.uni-wuerzburg.de
3. Department of Dermatology, Venerology and Allergology, University of Würzburg, Germany. trautmann_a@klinik.uni-wuerzburg.de
Abstract
Itching erythematous or eczematous plaques around injection sites are quite frequent side effects of heparin treatment and are clinical symptoms of a delayed-type hypersensitivity to heparins. In most cases, changing the subcutaneous therapy from unfractionated to low molecular weight heparin or treatment with heparinoids does not provide improvement, due to extensive cross-reactivity. Interestingly, it has been demonstrated that patients with delayed-type hypersensitivity to subcutaneously injected heparins tolerate intravenous application of heparin in controlled challenge tests. A patient with known delayed-type hypersensitivity to heparins received the heparinoid, danaparoid, subcutaneously for thrombosis prophylaxis after orthopedic surgery. After the first few injections, eczematous plaques developed; administration of the anticoagulant was continued and gradually resulted in generalized eczema despite treatment with topical and oral glucocorticoids. However, the patient required further anticoagulation. After discontinuation of subcutaneous injections and a switch to intravenous heparin, rapid improvement and clearing of skin lesions occured. Therefore, in cases of delayed-type hypersensitivity to subcutaneously injected heparins, the switch from subcutaneous to intravenous heparin administration may be justified.
Introduction
Immobilization after joint and bone surgery requires peri- and postoperative thrombosis prophylaxis. In most cases subcutaneously applicable unfractionated or low-molecular weight heparins are used for anticoagulation. Itching erythematous or eczematous plaques around injection sites are quite frequent side effects of heparin treatment and are the clinical symptoms of a delayed-type hypersensitivity to heparins [1]. The usual latency for development of characteristic lesions during ongoing therapy is 7 to 10 days; in the case of prior sensitization and re-exposure skin lesions appear within 1 to 3 days. The spectrum of skin lesions ranges from mild erythema with little infiltration to typical eczematous plaques with papulovesicles located on an infiltrated erythematous background. Less frequently, in cases with continuation of subcutaneous injections despite local reaction, a generalized eczema or exanthema with accentuation around injection sites may be observed [2, 3, 4]. Allergy testing of patients in these studies with a panel of different heparin preparations revealed extensive cross-reactivity among heparins that excluded a causal role of preservatives sometimes added to the heparin solution, such as sodium metabisulfite, benzyl alcohol, or chlorocresol.
In most cases, changing the subcutaneous therapy from unfractionated to low molecular weight heparin or treatment with heparinoids does not provide improvement, due to extensive cross-reactivity. Theoretically, intravenous heparin in sensitized individuals (i.e., patients with delayed-type hypersensitivity to subcutaneous heparins) may reach the skin via blood circulation and may trigger a hematogeneous generalized eczematous eruption. However, prior reports [5] and our previous studies [6, 7], show that patients with delayed-type hypersensitivity may tolerate intravenous application of heparin in controlled challenge tests at a later date.
Clinical synopsis
After intramedullary stabilization of a long spiral fracture of the humerus including the humeral head with a locking nail, a 69-year-old obese female (body mass index = 45) with a history of delayed-type hypersensitivity to heparins was administered subcutaneously the heparinoid danaparoid (Orgaran™, contains the preservative sodium metabisulfite) twice daily for deep vein thrombosis prophylaxis. One year prior she had experienced local eythema and pruritus after subcutaneous injections of enoxaparin (Clexane™, contains no preservative) and dalteparin (Fragmin™, contains no preservative), respectively. This time, only one day after the first injection of danaparoid she developed infiltrated erythema with papules and vesicles, consistent with allergic contact dermatitis, around the injection sites. The patient required further anticoagulation and the subcutaneous injections were continued, initially on the lower abdomen, and later on unaffected areas of the upper thighs. During the following days a generalized eczematous eruption developed that could be controlled neither by topical nor by oral glucocorticoids.
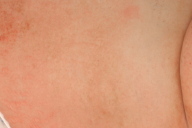
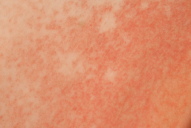
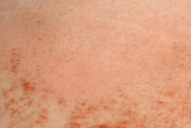
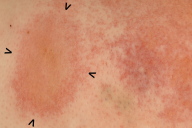
On postoperative day 6 the patient was transferred from the orthopedic surgery unit to the dermatology department. She exhibited a generalized eczema on her trunk and proximal extremities (Figs. 1a, 1b, 1c). Clinical examination at this time point, revealed injection sites on the upper thighs where eczematous plaques still had a round configuration (Fig. 1d).
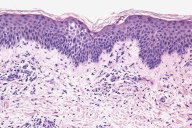
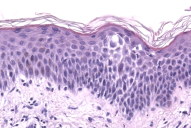
Histopathology revealed epidermal spongiosis accompanied by a superficial perivascular lymphocytic infiltrate (Figs. 2a, 2b).
 |  |
| Figure 1a | Figure 1b |
|---|
 |  |
| Figure 1c | Figure 1d |
|---|
Figure 1. 1a: Generalized eczematous eruption 6 days after initiation of anticoagulation therapy with subcutaneous danaparoid injections twice daily. 1b: Intertriginous regions show accentuation of eczematous skin inflammation, e.g., close-up of the submammary region. 1c: Danaparoid injections were administered for the first 3 days in the lower abdomen. On day 6 because of confluence and spreading of eczematous lesions on the lower abdomen, the initial single plaques around the injection sites could no longer be identified. 1d: Starting with day 4, danaparoid was injected into the upper thigh. Here, one injection site can still be discriminated despite confluence and spreading of eczema.
 |  |
| Figure 2a | Figure 2b |
|---|---|
| Figure 2a & 2b. Histopathology shows dermal lymphocytes infiltrating the spongiotic epidermis, giving rise to the eczematous clinical appearance. | |
Taking into account both the clinical presentation and the patient's previous allergy history, the diagnosis of delayed-type hypersensitivity to subcutaneous heparins and heparinoids was established. Considering our previous experience in patients with intravenous tolerance to heparins we decided to discontinue subcutaneous heparinoid injections and administer unfractionated heparin (Liquemin™) intravenously (15,000 IU/24h) without prior allergy testing. During intravenous heparin therapy and concomitant topical glucocorticoid application the eczema cleared within one week.
Discussion
Allergy to subcutaneously injected heparins is a common problem in the postoperative care of orthopedic patients who require anticoagulation due to immobilization. Alternative heparinoids were sought initially due to the complication of antibody-mediated heparin-induced thrombocytopenia. Although danaparoid has very low cross-reactivity with heparin for binding by heparin-induced antibody, it did cross react with heparin in the production of delayed-type hypersensitivity. Delayed-type hypersensitivity to heparins is ordinarily triggered by exposure of the skin due to subcutaneous injections. Female gender and obesity are risk factors for development of delayed-type hypersensitivity to heparins, implying hormonal and metabolic influences [8]. Intravenous heparin application in sensitized individuals may theoretically result in a generalized eczema. Interestingly, affected patients develop allergic symptoms only to subcutaneously administered heparins and heparinoids but tolerate intravenous administration [5, 6, 7]. One explanation for this phenomenon may be a non-specific binding of heparins to proteins and other macromolecules after subcutaneous injection. In this context, differences in presentation and processing of heparin antigens likely depends upon the application modality. However, the antigenic determinants of the heparin molecule have not been elucidated yet. Always, the most important condition in the differential diagnosis of erythematous and eczematous plaques after subcutaneous heparin treatment must be remembered: heparin-induced skin necrosis may initially resemble delayed-type hypersensitivity to heparin [9].
Due to post-operative immobilization and additional risk factors including obesity, anticoagulation for deep vein thrombosis prophylaxis was strictly required for our patient. To determine the best treatment regimen under these circumstances, there are several points that have to be considered. First, there is a high degree of cross-reactivity among all unfractionated and low molecular weight heparins. Therefore, a simple switch to another subcutaneous heparin preparation is not advisable. A generalized eczematous dermatitis is the sign of a high grade of sensitization to heparins and heparinoids, i.e., to anionic polysaccharides. Pentosanpolysulfat (a semisynthetic heparinoid) and the synthetic pentasaccharid, fondaparinux, are also anionic polysaccharides and cross-reactivity is therefore likely. Recombinant hirudines (lepirudin, desirudin, bivalirudin) and other direct thrombin inhibitors, such as argatroban, are potential alternatives to intravenous anticoagulation with heparins. However, the use of these alternative compounds may present a challenge because there are no antidotes available for cases of overdose and bleeding complications may occur [10]. Moreover, desirudin, bivalirudin, and argatroban have application restrictions and it is obvious that despite approval of new anticoagulants, heparins remain the medication of first choice [11].
This case report illustrates important aspects of delayed-type allergy to subcutaneously injected heparins and provides an approach to the management of affected patients in routine clinical settings:
1. In case of delayed-type hypersensitivity to heparins the high degree of cross-reactivity of various anionic polysaccharides, i.e., heparins and semisynthetic heparinoids, has to be considered.
2. Allergic hypersensitivity reactions are not all-or-nothing responses but may present as a spectrum of symptoms depending on the degree of sensitization. Patients with a rather low degree of sensitization may only develop erythematous plaques around injection sites. Severe sensitization, as in our case, begins as a pronounced local eczematous reaction However, continuation of heparin injections in such cases may lead to development of a generalized eruption.
3. Intravenous administration of heparin is usually tolerated by patients with delayed-type hypersensitivity to subcutaneously injected heparins and heparinoids. In urgent cases the intravenous use of heparin without prior allergy testing may be necessary and justified according to current data.
References
1. Klein GF, Kofler H, Wolf H, Fritsch PO. Eczema-like, erythematous, infiltrated plaques: a common side effect of subcutaneous heparin therapy. J Am Acad Dermatol. 1989 Oct;21(4 Pt 1):703-7. PubMed2. Estrada Rodriguez JL, Gozalo Reques F, Ortiz de Urbina J, Matilla B, Rodriguez Prieto MA, Gonzalez Moran NA. Generalized eczema induced by nadroparin. J Investig Allergol Clin Immunol. 2003;13(1):69-70. PubMed
3. Kim KH, Lynfield Y. Enoxaparin-induced generalized exanthem. Cutis. 2003 Jul;72(1):57-60. PubMed
4. Grassegger A, Fritsch P, Reider N. Delayed-type hypersensitivity and cross-reactivity to heparins and danaparoid: a prospective study. Dermatol Surg. 2001 Jan;27(1):47-52. PubMed
5. Boehncke WH, Weber L, Gall H. Tolerance to intravenous administration of heparin and heparinoid in a patient with delayed-type hypersensitivity to heparins and heparinoids. Contact Dermatitis. 1996 Aug;35(2):73-5. PubMed
6. Trautmann A, Brocker EB, Klein CE. Intravenous challenge with heparins in patients with delayed-type skin reactions after subcutaneous administration of the drug. Contact Dermatitis. 1998 Jul;39(1):43-4. PubMed
7. Gaigl Z, Pfeuffer P, Raith P, Brocker EB, Trautmann A. Tolerance to intravenous heparin in patients with delayed-type hypersensitivity to heparins: a prospective study. Br J Haematol. 2005 Feb;128(3):389-92. PubMed
8. Kroon C, de Boer A, Kroon JM, Schoenmaker HC, van den Meer FJ, Cohen AF. Influence of skinfold thickness on heparin absorption. Lancet. 1991 Apr 20;337(8747):945-6. PubMed
9. Warkentin TE, Roberts RS, Hirsh J, Kelton JG. Heparin-induced skin lesions and other unusual sequelae of the heparin-induced thrombocytopenia syndrome: a nested cohort study. Chest. 2005 May;127(5):1857-61. PubMed
10. Kam PC, Kaur N, Thong CL. Direct thrombin inhibitors: pharmacology and clinical relevance. Anaesthesia. 2005 Jun;60(6):565-74. PubMed
11. Hyers TM. Management of venous thromboembolism: past, present, and future. Arch Intern Med. 2003 Apr 14;163(7):759-68. PubMed
© 2008 Dermatology Online Journal

