Facial acneiform rash associated with sorafenib
Published Web Location
https://doi.org/10.5070/D34rg64357Main Content
Facial acneiform rash associated with sorafenib
Beatriz Fleta-Asín MD1 Sergio Vañó-Galván MD1, Alejandro Ledo-Rodríguez MD2, Maite Truchuelo-Díez MD1, Pedro Jaén-Olasolo MD PhD1
Dermatology Online Journal 15 (4): 7
1. Dermatology Department2. Gastroenterology Department
Ramon y Cajal Hospital, University of Alcalá de Henares, Madrid, Spain. beatriz_fleta@hotmail.com
Abstract
Sorafenib is an oral multikinase inhibitor that is useful in the treatment of various solid cancer. We report a patient who developed an acneiform facial eruption during treatment with this medication.
Introduction
Tyrosine kinase (TK) and epidermal growth factor receptor (EGFR) inhibitors have recently emerged as new drugs for cancer treatment. In contrast to conventional chemotherapy, which acts on dividing cells generating toxic effects on normal tissues, TK and EGFR-inhibitors act on a subpopulation of cells, directly involved in tumor progression, that exhibit certain overexpressed cellular pathways. With the use of these compounds new toxicities are emerging, usually skin toxicities [1].
Sorafenib is an oral multikinase inhibitor active on solid cancer. Although skin toxicity has been reported, acneiform rash is considered a less common skin adverse event, although it has been widely reported in association with EGFR inhibition [2]. We report a case of a patient who developed an acneiform facial rash during treatment with sorafenib.
Case report
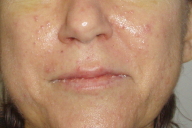
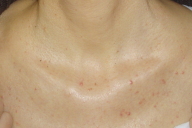
A 42-year-old woman diagnosed with liver epithelioid hemangioendothelioma presented with a 10-day history of asymptomatic skin eruption. She underwent liver transplantation one year before, but six months later tumor relapse was detected. Therefore, therapy with sorafenib was started at a dosage of 400 mg bid. Four weeks later sorafenib dosage was increased to 800 mg BID; the day after she developed an acneiform facial eruption consisting of papules and pustules along face and upper trunk, without mucous membrane involvement (Figs. 1 & 2). Owing to an inadequate tumor response and suspected involvement in the acneiform rash, the sorafenib dosage was reduced to 400 mg BID; the acneiform eruption resolved almost completely within two weeks. Disease advanced and the patient died two months later.
 |  |
| Figure 1 | Figure 2 |
|---|---|
| Figure 1. Facial papules and pustules Figure 2. Papules and pustules in upper trunk | |
Molecules controlling cell proliferation and death, such as TK inhibitors (TKI) and EGFR blockers, are a new target in the strategy in treatment of human cancer. Among TKI, sorafenib has been approved by the Food and Drug Administration for inoperable liver cancer and advanced renal cell carcinoma and sunitinib for advanced renal carcinoma and gastrointestinal stromal tumors [3, 4].
Sorafenib is an orally available multikinase inhibitor active on vascular endothelial growth factor receptor-2 and 3, platelet-derived growth factor receptor-beta, B-RAF, C-RAF, flt3 and C-Kit. Such simultaneous interference with multiple TK might be more effective than single target agents. Although sorafenib has an excellent safety profile, more than 90 percent of patients develop skin reactions like seborrheic dermatitis-like rash, pruritus, erythema, xerosis, stomatitis, subungual splinter hemorrhages, alopecia, modification of hair growth or pigmentation, skin discoloration, and hand-foot skin reaction [5, 6].
Acneiform eruption is a well-known cutaneous side effect of EGFR blockers, but has been rarely reported in relation with sorafenib. We have performed a Medline search in order to detect association between sorafenib and acneiform eruption. To our knowledge, scarce data based on case reports or patient series have been provided [2, 7].
In addition, although cutaneous side effects of TKI and EGFR blockers are reported to have a latency from seven days to three months [8], acneiform eruption developed in our patient about 24 hours after increasing the sorafenib dosage and was responsive to dose adjustment.
Our case supports that facial acneiform eruption must be considered one of the skin side effects induced by sorafenib. The fact that it is also associated with EGFR blockers suggests overlapping molecular pathways for EGFR blockers and TKI or a non-specific role of these drugs on a particular cutaneous symptom [8]. Pathogenic explanation remains complex. Epidermal growth factor receptor is a transmembrane protein receptor expressed in epidermal and follicular keratinocytes, sebaceous and eccrine epithelium and various connective tissue cells; it is involved in normal epidermal processes [9]. It has a tyrosine kinase domain in the intracellular part of the receptor responsible for the phosphorylation of downstream signalling proteins [10]. One of the best-characterized is the Raf/MEK/ERK signalling pathway, which is involved in cell-cycle regulation and proliferation. Sorafenib, as a multikinase inhibitor, targets serine-threonine kinases and tyrosine kinase receptor, which modifies the Raf/MEK/ERK pathway, leading to similarities in skin reactions between EGFR inhibitors and TKIs [11].
The therapeutic approach for acneiform eruption varies according to severity. Experience in management is limited to mainly empirical measures. Preventive measures have not been developed because the acneiform eruption responds well to local or oral therapy, is often temporary, diminishes in intensity with exposure, and is rarely dose or treatment limiting [12]. Preventive treatment with topical 4 percent erythromycin emulsion and oral fusidic acid was associated with facial erythema without papules or pustules in two patients under therapy with cetuximab, an EFGR blocker [13]. Intranasal mupirocin has been considered to reduce the likelihood of secondary infection [9].
In summary, acneiform facial eruption should be considered as a potential cutaneous side effect of sorafenib. It remains to be elucidated if the acneiform eruption and clinical outcome are related in the case of sorafenib, as seems true with EGFR-inhibitors. A better understanding of these drugs and mechanisms of action is needed to clarify skin reactions and associations with tumor response.
References
1. Zureikat AH, McKee MD. Targeted therapy for solid tumors: current status. Surg Oncol Clin N Am. 2008 Apr;17(2):279-301, vii-viii. [PubMed]2. Porta C, Paglino C, Imarisio I, Bonomi L. Uncovering Pandora's vase: the growing problem of new toxicities from novel anticancer agents. The case of sorafenib and sunitinib. Clin Exp Med. 2007 Dec;7(4):127-34. [PubMed]
3. U.S. Food and drug administration. Drug information. Nov 20, 2007; April 4, 2009. FDA Approves Sorafenib (Nexavar) for the Treatment of Unresectable Hepatocellular Carcinoma. FDA, Rockville, Maryland. Available in: http://www.fda.gov/CDER/Offices/OODP/whatsnew/sorafenib.htm
4. U.S. Food and drug administration. Drug information. January 26, 2006; April 4, 2009 FDA Approves New Treatment for Gastrointestinal and Kidney Cancer. FDA, Rockville, Maryland. Available in: http://www.fda.gov/bbs/topics/news/2006/new01302.html
5. Yang CH, Lin WC, Chuang CK, Chang YC, Pang ST, Lin YC, Kuo TT, Hsieh JJ, Chang JW. Hand-foot skin reaction in patients treated with sorafenib: a clinicopathological study of cutaneous manifestations due to multitargeted kinase inhibitor therapy. Br J Dermatol. 2008 Mar;158(3):592-6. [PubMed]
6. Lacouture ME, Wu S, Robert C, Atkins MB, Kong HH, Guitart J, Garbe C, Hauschild A, Puzanov I, Alexandrescu DT, Anderson RT, Wood L, Dutcher JP. Evolving strategies for the management of hand-foot skin reaction associated with the multitargeted kinase inhibitors sorafenib and sunitinib. Oncologist. 2008 Sep;13(9):1001-11. [PubMed]
7. Alexandrescu DT, Vaillant JG, Dasanu CA. Effect of treatment with a colloidal oatmeal lotion on the acneform eruption induced by epidermal growth factor receptor and multiple tyrosine-kinase inhibitors. Clin Exp Dermatol. 2007 Jan;32(1):71-4. [PubMed]
8. Robert C, Soria JC, Spatz A, Le Cesne A, Malka D, Pautier P, Wechsler J, Lhomme C, Escudier B, Boige V, Armand JP, Le Chevalier T. Cutaneous side-effects of kinase inhibitors and blocking antibodies. Lancet Oncol. 2005 Jul;6(7):491-500. [PubMed]
9. Pérez-Soler R, Delord JP, Halpern A, Kelly K, Krueger J, Sureda BM, von Pawel J, Temel J, Siena S, Soulières D, Saltz L, Leyden J. Oncologist 2005 May;10(5):345-56. HER1/EGFR inhibitor-associated rash: future directions for management and investigation outcomes from the HER1/EGFR inhibitor rash management forum. [PubMed]
10. Widakowich C, de Castro G Jr, de Azambuja E, Dinh P, Awada A. Review: side effects of approved molecular targeted therapies in solid cancers. Oncologist. 2007 Dec;12(12):1443-55. [PubMed]
11. Moore M, Hirte HW, Siu L, Oza A, Hotte SJ, Petrenciuc O, Cihon F, Lathia C, Schwartz B. Phase I study to determine the safety and pharmacokinetics of the novel Raf kinase and VEGFR inhibitor BAY 43-9006, administered for 28 days on/7 days off in patients with advanced, refractory solid tumors. Ann Oncol. 2005 Oct;16(10):1688-94. 8. [PubMed]
12. Galimont-Collen AF, Vos LE, Lavrijsen AP, Ouwerkerk J, Gelderblom H. Classification and management of skin, hair, nail and mucosal side-effects of epidermal growth factor receptor (EGFR) inhibitors. Eur J Cancer. 2007 Mar;43(5):845-51. [PubMed]
13. Jacot W, Bessis D, Jorda E, Ychou M, Fabbro M, Pujol JL, Guillot B. Acneiform eruption induced by epidermal growth factor receptor inhibitors in patients with solid tumours. Br J Dermatol. 2004 Jul;151(1):238-41. [PubMed]
© 2009 Dermatology Online Journal

