Lichen planopilaris and psoriasis
Published Web Location
https://doi.org/10.5070/D34pt100txMain Content
Lichen planopilaris and psoriasis
Tameka K Lane MD, Hideko Kamino MD, Ruth F Walters MD, Shane Meehan MD, Miriam K Pomeranz MD
Dermatology Online Journal 14 (10): 4
Department of Dermatology, New York UniversityAbstract
A 34-year-old woman presented with large, scaly patches of alopecia with a peripheral rim of violaceous, folliculocentric papules and appreciable pruritus of one-year duration. Histopathologic examination showed changes consistent with lichen planopilaris and psoriasis, which was suggested by neutrophilic spongiosis. Consequently, cyclosporine and betamethasone valerate topical 0.12 percent foam twice daily were initiated. A short time after, there was clinical reduction of perifollicular erythema and attenuation of pruritus. However, there was no decrease of scale. Although LLP is classified in the lymphocytic group of cicatricial alopecias, this case demonstrates a clinical and histopathologic overlap with a psoriasiform dermatosis which may represent a collision of two diseases.
History
A 34-year-old woman presented to New York University Dermatological Associates office for evaluation and treatment of hair loss and scaly scalp of one year's duration. Prior to her initial presentation, a diagnosis of psoriasis of the scalp had been made and treated with several topical therapies without diminution of symptoms. On review of systems she only noted blurred vision of right eye for which she undergoing ophthalmologic evaluation. Two weeks after her initial visit, she returned complaining of substantially marked increase of hair loss quantity and worsening of pruritus. After review of laboratory and histopathologic data obtained on initial visit, cyclosporine was initiated at 3mg/kg in divided doses twice daily in conjunction with betamethasone valerate 0.12 percent foam twice daily. In the succeeding two weeks, she reported decreased pruritus and a reduction of perifollicular erythema; however, scale did not diminish.
 |  |
| Figure 1 | Figure 2 |
|---|
 |
| Figure 3 |
|---|
Physical Examination
On the bilateral parietal areas and crown of scalp were large, scaly patches of alopecia with peripheral rim of violaceous, folliculocentric papules and macules.
Lab
A complete blood count and a comprehensive metabolic panel were normal.
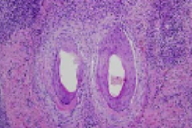
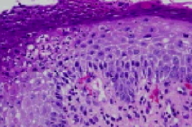
Histopathology
The epidermis exhibits acanthosis, diminution of the granular layer, spongiform subcorneal pustules and parakeratosis with neutrophils. There is a superficial and deep, perivascular and perifollicular infiltrate of lymphocytes, plasma cells, neutrophils, and eosinophils. There is clefting between the follicular epithelium and the peri-infundibular fibrosis. Periodic acid-Schiff-D and Gram stains fail to show fungi and bacteria.
Comment
Lichen planopilaris (LPP), initially described in 1895 by Pringle [1], is a rare inflammatory cicatricial alopecia of unknown etiology. LPP is predominantly a disease of adulthood and typically presents in middle age [2, 3]. In contrast to lichen planus, LPP is more frequent in women. While only 17 percent to 28 percent of patients at time of presentation exhibit lichen planus elsewhere on the body, approximately one-half of patients develop classic lichen planus at some point during their disease course [4]. There are a variety of presenting symptoms for LPP; the most common include severe scalp pruritus, hair loss, scalp tenderness, and scaling [2, 5]. The classic clinical hallmark features include white, atrophic patches of alopecia with the absence of follicular ostia. Residual hairs at the expanding margins demonstrate perifollicular erythema, scale, and hyperkeratotic papules. While any scalp location may be involved, the frontal-central, crown, and parietal scalp are most commonly affected [2, 5, 6]. A positive pull test for anagen hairs is useful for distinguishing active disease and delineating a histopathologic representative biopsy site [7]. Some authors advocate that scalp investigations of cicatricial alopecias include two punch biopsies: one submitted for travserse sectioning and the other bisected for vertical sectioning and direct immunofluorescence [8].
Histopathologic features of LPP include a predominant lymphocytic, band-like, perifollicular infiltrate that involves the follicular infundibulum and the isthmus. Frequently there are necrotic keratinocytes and vacuolar degeneration of the outer root sheath basal layer. In advanced cases, there is a characteristic replacement of follicles by prominent, vertical, fibrous tracts with degenerated elastic fiber clumps [9, 10]. It is postulated that inflammation targets the hair, bulge, which is the site of stem cells and results in the loss of hair re-growth potential [11].
Treatment of LPP is difficult because there have not been any proved effective therapies. Therefore, realistic therapeutic expectations should not be for cure but rather amelioration of symptoms and thwarting disease progression. Early aggressive intervention is suggested to minimize permanent scars. Reported treatments vary widely from vitamin supplements to those requiring judicious monitoring; however, they all lack evidenced-based substantiation, in part due to the rarity of the disease entity. Cited treatments include ketoconazole shampoo, biotin supplements, dapsone [12], topical glucocorticoids [13], intralesional glucocorticoids [5, 13], minoxidil, tetracycline, antimalarials, cyclosporine, and isotretinoin [14].
While scale is a salient feature of LPP, our patient displayed a prolific quantity of scale. The exuberant scale, which when combined with the histopathologic finding of neutrophilic spongiosis, allowed consideration of seborrheic dermatitis or psoriasis as an additional diagnosis. This patient had been told she had psoriasis prior to a period of hair loss that eventuated in the biopsy. Psoriasis or seborrheic dermatitis may have antedated her development of LPP and may have obscured the clinical picture whenLPP and psoriasiform dermatitides.
References
1. Pringle JJ. Cited by: Adamson HG. Folliculitis decalvans et atrophicans: report of a case. Br J Dermatol 1905; 17:772. Tan E, et al. Primary cicatricial alopecias: clinicopathology of 112 cases. J Am Acad Dermatol 2004; 50: 25 PubMed
3. Chieregato C, et al. Lichen planopilaris: report of 30 cases and review of the literature. Int J Dermatol 2003; 42: 342 PubMed
4. Eisen D. The evaluation of cutaneous, genital, scalp, nail, esophageal, and ocular involvement in patients with oral lichen planus. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 1999; 88: 431 PubMed
5. Mehregan DA, et al. Lichen planopilaris: clinical and pathologic study of forty-five patients. J Am Acad Dermatol 1992; 27: 935 PubMed
6. Matta M, et al. Lichen planopilaris: a clinicopathologic study. J Am Acad Dermatol 1990; 22: 594 PubMed
7. Mirmirani P, et al. Short course of oral cyclosporine in lichen planopilaris, J Am Acad Dermatol 2003; 49: 667 PubMed
8. Ross EK, et al. Update on primary cicatricial alopecias. J Am Acad Dermatol 2005; 53: 1 PubMed
9. Sperling LC. Scarring alopecia and the dermatopathologist. J Cutan Pathol 2001; 28: 333 PubMed
10. Mirmirani P, et al. Primary cicatricial alopecia: histopathologic findings do not distinguish clinical variants. J Am Acad Dermatol 2005; 52: 637 PubMed
11. Mobini N, et al. Possible role of the bulge region in the pathogenesis of inflammatory scarring alopecia: lichen planopilaris as the prototype. J Cutan Pathol 2005; 32: 675 PubMed
12. Cevasco NC, et al. A case-series of 29 patients with lichen planopilaris: the Cleveland Clinic Foundation experience on evaluation, diagnosis, and treatment. J Am Acad Dermatol 2007; 57: 47 PubMed
13. Whiting DA. Cicatricial alopecia: clinico-pathological findings and treatment. Clin Dermatol 2001; 19: 211 PubMed
14. Mirmirani P, et al. Short course of oral cyclosporine in lichen planopilaris. J Am Acad Dermatol 2003; 49: 667 PubMed
© 2008 Dermatology Online Journal

