Acquired cutis laxa in a 55-year-old female with multiple myeloma and serologic evidence of systemic lupus erythematosus
Published Web Location
https://doi.org/10.5070/D34dj0g7brMain Content
Acquired cutis laxa in a 55-year-old female with multiple myeloma and serologic evidence of systemic lupus erythematosus
Dennis P Kim1, Peter A Klein MD2
Dermatology Online Journal 17 (7): 8
1. School of Medicine, Stony Brook University, Stony Brook, New York. dennis.kim@stonybrook.edu2. Department of Dermatology, Stony Brook University, Stony Brook, New York
Abstract
Cutis laxa (CL) is a rare connective tissue disorder characterized by loosely hanging skin folds. Histopathology reveals degenerative changes in the dermal elastic fibers, although loss of elastin can also occur in alveolar walls, blood vessels, and other organs. The coexistence of autoimmune diseases and monoclonal gammopathies is rare but well documented in the literature. Here we report an unusual case of cutis laxa (CL) preceding the development of serologic evidence of systemic lupus erythematosus (SLE) and a diagnosis of multiple myeloma (MM) by seven and eleven years respectively.
Case report
 |  |
| Figure 1 | Figure 2 |
|---|---|
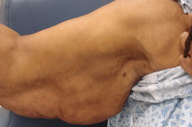
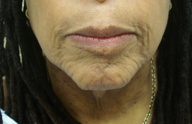
| Figure 1: Loose, redundant skin hanging in pendulous folds Figure 2. Lax and wrinkled skin on the face and neck | |
A 55-year-old woman presented to us with a ten year history of loosening of her skin. The loosening started under her arms in 1998 and had progressed in recent years to include her face, neck, and legs. She had no history of an inflammatory process involving the skin preceding the development of the loose folds and the patient had no family history of any skin disorder. However, in the years following the onset of her dermatologic manifestations, she had multiple hemato-oncologic, renal, and rheumatologic evaluations for abnormalities that corresponded temporally with worsening of her dermatosis.
In 2005, seven years after the onset of her skin abnormalities, she was evaluated for persistent hematuria and proteinuria. Serum analysis identified an IgG-κ monoclonal gammopathy and kidney biopsy showed membranous glomerular nephritis associated with IgG-κ deposition. Subsequent bone marrow testing revealed 10 percent plasma cells in the setting of leukopenia and anemia. She was diagnosed with monoclonal gammopathy of undetermined significance (MGUS) and her cytopenia responded well to corticosteroid treatment. Laboratory testing revealed a positive ANA at 1:160 dilution with a speckled pattern, positive dsDNA at 70 IU/mL, and hypocomplementemia. These findings, in light of a persistent anemia, raised the possibility of systemic lupus erythematosus.
In 2008, she presented to us with recent progression of her skin laxity and, after a full clinical evaluation, punch biopsy confirmed her clinical diagnosis of acquired cutis laxa. A few months later, worsening anemia and proteinuria prompted repeat bone marrow testing showing progressive plasmacytosis. A second kidney biopsy found immune-mediated glomerular nephritis and monoclonal immunoglobulin deposition disease (MIDD). She was diagnosed with kappa light chain multiple myeloma (MM) and began treatment with lenalidomide and low-dose dexamethasone with plans for future stem cell collection and subsequent transplantation. She had a good response in terms of reduction of her free light chain disease. However, she was eventually removed from the protocol owing to persistent cytopenia. Stem cell mobilization was attempted on two separate occasions with mozobil and G-CSF, but insufficient stem cells were collected both times.
The patient was then put on a regimen of bortezomib and dexamethasone in 2010 and had an excellent response with bone marrow biopsy showing 1 percent plasma cells and no definite evidence of light chain restriction. However, her course was again complicated by persistent cytopenias and treatment was discontinued a few months later. Because her cytopenia did not improve when her multiple myeloma was at a minimum, and given her prior history of positive SLE serology, she was once again evaluated by a rheumatologist and also began treatment directed at immune-mediated processes. The rheumatologic evaluation was not definitive. However, treatment with IVIG and danazol for the first two months have thus far stabilized her blood counts and she is clinically better.
Physical examination
Cutaneous exam revealed large, pendulous areas of sagging skin on the upper arms bilaterally (Figure 1). The skin on the dorsum of her hands appeared mildly lax. Large areas of loose skin were also present on the thighs bilaterally. The jowls had a hound-dog facies appearance (Figure 2).
Histopathology
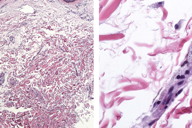
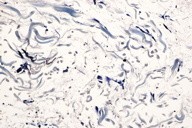
Punch biopsy revealed loosely arranged collagen bundles in the upper third of the dermis accompanied by interstitial foamy macrophages. Acid Orcein-Giemsa stain showed diminished elastic fibers within this portion of the dermis and also diminished numbers in the remainder of the reticular dermis (Figure 3). Orcein stain also demonstrates diminished elastic fibers throughout the dermis (Figure 4).
Discussion
Cutis laxa is a rare connective tissue disorder caused by defects in the elastic fiber network affecting multiple tissues, predominantly the skin. It is characterized clinically by loose, pendulous skin giving a characteristic hound-dog facies. Histologically there is loss of elastic tissue in the papillary and reticular dermis [1]. Loss of elastin can also occur in alveolar walls, blood vessels, and various other organs leading to systemic involvement. Cutis laxa may be acquired or inherited as a dominant, recessive, or X-linked recessive disease [2, 3]. The acquired form may develop at any age, but usually occurs in the second and third decade of life, with no evidence of internal organ involvement.
The etiology of CL is not completely understood. Electron microscopic studies suggest that loss and fragmentation of elastic fibers lead to sagging skin with reduced elasticity and resilience without scarring because collagen fibers are usually not affected. Proposed pathogenic mechanisms include excess elastase activity, dysfunction of elastase inhibitors, copper deficiency, decreased lysyl oxidase activity, or an immune-mediated mechanism [4].
Approximately one-half of the cases of acquired cutis laxa are associated with prior infection or drug medication and termed post-inflammatory elastolysis and cutis laxa (PECL) [5]. Post-inflammatory elastolysis and cutis laxa is characterized by an urticarial or papular eruption followed by acute destruction of elastic tissue that results in atrophy and disfigurement. Systemic manifestations often accompany the inflammation and include fever, malaise, and leukocytosis. The cutaneous laxity that follows is limited to areas of previous inflammation [6]. Post-inflammatory elastolysis and cutis laxa usually affects girls 1 to 4 years of age of African descent and produces a fine wrinkling, resulting in an aged appearance in these young children. Finally, in contrast to other forms of elastolysis, there are degenerative changes of both elastic and collagen fibers in PECL [7].
There is no satisfactory medical treatment for CL. Dapsone has been successful in controlling swelling during the inflammatory phase of the disease. It is thought to act on neutrophil infiltrates often associated with CL by suppressing calcium-dependent elastase release [8]. Plastic surgical repair seems to be the only strategy to confer physical, psychological, and social benefit for patients with CL. In addition to changes in the skin, there is often systemic involvement predominately affecting the lungs (emphysema) [9]. However, cardiovascular (aortic aneurysm, mitral valve prolapse), gastrointestinal (hiatal and inguinal hernias), and genitourinary abnormalities can also be seen. Patients should be evaluated for internal organ involvement and regular cardiac monitoring is recommended to avert a potentially fatal aortic rupture.
Only fifteen cases of acquired CL associated with a plasma cell dyscrasia have been reported in the literature since the association was first described by Scott et al in 1976 [10]. Eleven are associated with MM, two with heavy chain disease, one with light chain disease, and one with MGUS [10-23]. Because of the rarity of these disorders, various linkages between plasma cell dyscrasias and CL have been postulated. Several case reports use direct immunofluorescence and electron microscopy to demonstrate binding of monoclonal antibodies to elastic fibers [11, 16, 17]. Whereas these findings strengthen the evidence for an immune mediated mechanism for elastolysis, other reports of CL associated with MM lack this same immunoglobulin deposition [18]. Also, there does not seem to be a correlation between the level of monoclonal paraprotein in the serum and the severity of the acquired CL [16].
In addition to the association between CL and MM, both of these conditions have independently been linked with SLE. Cutis laxa-like degeneration of dermal elastic fibers was first observed in patients with SLE in 1972 [25]. Since then, several case reports have shown a correlation between SLE and middermal elastolysis [24, 25, 26]. The coexistence of SLE and MM has only been reported twelve times, but the association between SLE and lymphoproliferative malignancies is well known [27, 28, 29, 30].
To our knowledge, no cases have ever been reported of CL, MM, and SLE occurring simultaneously. The classification system devised by the American College of Rheumatology delineates eleven criteria for the identification of SLE patients. If at least four are present, serially or simultaneously, during any interval of observation, a person can be said to have SLE [31]. Retrospective examination of our patient’s medical record reveals she met the requisite four criteria serially, over the course of five years (persistent proteinuria, leukopenia, anti-ANA, anti-dsDNA). Our patient had several rheumatologic evaluations by different physicians and, perhaps because she never presented with the criteria simultaneously, she has at this point not been diagnosed with SLE. This is the first reported case of CL with MM in the setting of a positive SLE classification and provides a clinical example of a possible pathophysiologic link between the three diseases.
References
1. Turner RB, Haynes HA, Granter SR, Miller DM. Acquired cutis laxa following urticarial vasculitis associated with IgA myeloma. J Am Acad Dermatol 2009 Jun 1;60(6):1052-7. [PubMed]2. Beighton P. The dominant and recessive forms of cutis laxa. J Med Genet 1972 Jun 1;9(2):216-21. [PubMed]
3. Byers PH, Siegel RC, Holbrook KA, Narayanan AS, Bornstein P, Hall JG. X-linked cutis laxa: defective cross-link formation in collagen due to decreased lysyl oxidase activity. N Engl J Med 1980 Jul 10;303(2):61-5. [PubMed]
4. Riveros CJP, Gavilán MFB, França LFS, Sotto MN, Takahashi MDF. Acquired localized cutis laxa confined to the face: case report and review of the literature. Int J Dermatol 2004 Dec 1;43(12):931-5. [PubMed]
5. Kerl H, Burg G, Hashimoto K. Fatal, penicillin-induced, generalized, postinflammatory elastolysis (cutis laxa). Am J Dermatopathol 1983 Jun 1;5(3):267-76. [PubMed]
6. Koch SE, Williams ML. Acquired cutis laxa: case report and review of disorders of elastolysis. Pediatr Dermatol 1985 Jul;2(4):282-8 [PubMed]
7. Verhagen AR, Woerdeman MJ. Post-inflammatory elastolysis and cutis laxa. Br J Dermatol 1975 Feb 1;92(2):183-90. [PubMed]
8. Suda T, Suzuki Y, Matsui T, Inoue T, Niide O, Yoshimaru T, Suzuki H, Ra C, Ochiai T. Dapsone suppresses human neutrophil superoxide production and elastase release in a calcium-dependent manner. Br J Dermatol 2005 May 1;152(5):887-95. [PubMed]
9. Gambichler T. Mid-dermal elastolysis revisited. Arch Dermatol Res 2010 Mar 1;302(2):85-93. [PubMed]
10. Scott MA, Kauh YC, Luscombe HA. Acquired cutis laxa associated with multiple myeloma. Arch Dermatol 1976 Jun 1;112(6):853-5. [PubMed]
11. Appiah YE, Onumah N, Wu H, Elenitsas R, James W. Multiple myeloma-associated amyloidosis and acral localized acquired cutis laxa. J Am Acad Dermatol 2008 Feb 1;58(2 Suppl):S32-3. [PubMed]
12. Cho SY, Maguire RF. Multiple myeloma associated with acquired cutis laxa. Cutis 1980 Aug 1;26(2):209-11. [PubMed]
13. Frémont G, Kérob D, Prost-Squarcioni C, Lièvre N, Rivet J, Tancrède E, Servant J-M, Fermand J-P, Morel P, Lebbé C. [Acquired cutis laxa and myeloma: large vacuolated cells in the dermis]. Ann Dermatol Venereol 2007 Jan 1;134(6-7):548-51. [PubMed]
14. Gupta A, Helm TN. Acquired cutis laxa associated with multiple myeloma. Cutis 2002 Feb 1;69(2):114-8. [PubMed]
15. Krajnc I, Rems D, Vizjak A, Hödl S. [Acquired generalized cutis laxa with paraproteinemia (IgG lambda). Immunofluorescence study, clinical and histologic findings with review of the literature]. Hautarzt 1996 Jul 1;47(7):545-9. [PubMed]
16. Maruani A, Arbeille B, Machet M-C, Barbet C, Laure B, Martin L, Machet L. Ultrastructural demonstration of a relationship between acquired cutis laxa and monoclonal gammopathy. Acta Derm Venereol 2010 Jul 1;90(4):406-8. [PubMed]
17. McCarty MJ, Davidson JM, Cardone JS, Anderson LL. Cutis laxa acquisita associated with multiple myeloma: a case report and review of the literature. Cutis 1996 Apr 1;57(4):267-70. [PubMed]
18. Nikko A, Dunnigan M, Black A, Cockerell CJ. Acquired cutis laxa associated with a plasma cell dyscrasia. Am J Dermatopathol 1996 Oct 1;18(5):533-7. [PubMed]
19. Tan S, Pon K, Bargman J, Ghazarian D. Generalized cutis laxa associated with heavy chain deposition disease. J Cutan Med Surg 2003 Jan 1;7(5):390-4. [PubMed]
20. Ting HC, Foo MH, Wang F. Acquired cutis laxa and multiple myeloma. Br J Dermatol 1984 Mar 1;110(3):363-7. [PubMed]
21. Turner RB, Haynes HA, Granter SR, Miller DM. Acquired cutis laxa following urticarial vasculitis associated with IgA myeloma. J Am Acad Dermatol 2009 Jun 1;60(6):1052-7. [PubMed]
22. Yoneda K, Kanoh T, Nomura S, Ozaki M, Imamura S. Elastolytic cutaneous lesions in myeloma-associated amyloidosis. Arch Dermatol 1990 May 1;126(5):657-60. [PubMed]
23. New HD, Callen JP. Generalized Acquired Cutis Laxa Associated With Multiple Myeloma With Biphenotypic IgG-lamba and IgA-kappa Gammopathy Following Treatment of a Nodal Plasmacytoma. Arch Dermatol 2011 Mar 1;147(3):323-8. [PubMed]
24. Boyd AS, King Jr LE. Middermal elastolysis in two patients with lupus erythematosus. Am J Dermatopathol 2001 Apr 1;23(2):136-8. [PubMed]
25. Schmitt D, Thivolet J, Perrot H. Ultrastructural study of the cutaneous elastic fibres in lupus erythematosus. Br J Dermatol 1972 Oct 1;87(4):355-60. [PubMed]
26. Alvarez-Cuesta CC, Raya-Aguado C, Fernández-Rippe ML, Sánchez TS, Pérez-Oliva N. Anetoderma in a systemic lupus erythematosus patient with anti-PCNA and antiphospholipid antibodies. Dermatology (Basel)2001 Jan 1;203(4):348-50. [PubMed]
27. Choi JW, Han SW, Kwon KT, Kim GW. Early onset multiple myeloma in a patient with systemic lupus erythematosus: a case report and literature review. Clin Rheumatol 2010 Nov 1;29(11):1323-6. [PubMed]
28. Okoli K, Irani F, Horvath W. Multiple myeloma and systemic lupus erythematosus in a young woman. J Clin Rheumatol 2009 Sep 1;15(6):292-4. [PubMed]
29. Bernatsky S, Boivin JF, Joseph L, Rajan R, Zoma A, Manzi S, et al. An international cohort study of cancer in systemic lupus erythematosus. Arthritis Rheum 2005 May 1;52(5):1481-90. [PubMed]
30. Ali YM, Urowitz MB, Ibanez D, Gladman DD. Monoclonal gammopathy in systemic lupus erythematosus. Lupus 2007 Jan 1;16(6):426-9. [PubMed]
31. Hochberg MC. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum 1997 Sep 1;40(9):1725. [PubMed]
© 2011 Dermatology Online Journal