Role of apoptosis stimulus factor and its ligand in the induction of apoptosis in some ultraviolet induced diseases
Published Web Location
https://doi.org/10.5070/D34ch1f2p5Main Content
Role of apoptosis stimulus factor and its ligand in the induction of apoptosis in some ultraviolet-induced diseases
Olfat Shaker 1, Randa Youssef 2
Dermatology Online Journal 12 (3): 4
Departments of Medical Biochemistry1 and Dermatology2, Faculty of Medicine, Cairo University, Egypt. olfat_shaker@hotmail.comAbstract
Background: Fas (factor of apoptosis stimulus) is one of the death receptors belonging to the tumor necrosis factor superfamily of receptors.
When bound to its ligand, Fas-ligand (Fas-L), it triggers apoptosis. Ultraviolet (UV) rays can induce keratinocyte apoptosis
by Fas/Fas-L interaction.
Aim: The aim of the study was to evaluate the role of Fas and Fas-L in basal cell carcinoma (BCC) as an example of malignant
neoplasm and discoid lupus erythematosus (DLE) as a benign skin disease, which are both induced by UV.
Subjects and methods: The study included 20 cases of BCC, 20 cases of DLE and ten control cases. All biopsies of BCC and DLE were examined histopathologically.
They were also examined for Fas and Fas-L by PCR.
Results: In BCC, apoptosis was detected in 60 percent of cases. Fas was found to be positive in only one case and it was found to
be negative in the other 19 cases (95 %). Fas-L was found to be positive in 100 percent of cases. In DLE, apoptosis was detected
in 90 percent of cases. Fas was positive in 80 percent of cases, Fas-L was positive in 90% of cases.
Conclusion: Over-expression of Fas-L and lack of expression of Fas by tumor cells together with other factors act in favor of BCC by
helping its survival and progression. Also, it seems that Fas/Fas-L interaction plays a critical role in the apoptosis seen
in cases of DLE and hence in the pathogenesis of DLE.
Introduction
Fas (Apo1/CD95) is one of the death receptors belonging to the TNF super family of receptors. Binding of the death ligand to the specific death receptors triggers apoptosis. The specific death ligand for Fas is Fas-ligand (Fas-L) [1]. Fas and Fas-L are required for the down-regulation of immune responses through activation-induced cell death [2].
Ultraviolet rays can induce keratinocyte apoptosis by Fas/Fas-L interaction or by direct aggregation and activation of Fas independently of Fas-L [3]. Ultraviolet treatment of cultured keratinocytes produced a phenotype Fas negative and Fas-L positive. This loss of Fas expression correlates with decreased sensitivity to Fas-L mediated apoptosis of mutated keratinocytes and thus contributing to tumorogenesis [4].
Basal cell carcinoma (BCC) is a locally malignant, sun-induced tumor [5]. The tumor cells in BCC express Fas-L with failure to express Fas, thus creating optimal regional immunosuppressive environment. This Fas-L expression by BCC is associated with Fas expression on peritumoral lymphocytes. Binding of Fas on peritumoral T-lymphocytes and Fas-L on tumor cells ends in apoptosis of Fas-bearing cells (immunoregulatory T-lymphocytes), which may predispose to the induction and progression of BCC [6].
Discoid lupus erythematosus (DLE) is a relatively benign autoimmune disorder of the skin, most frequently involving the sun-exposed areas [7]. Fas was found to be expressed in the epidermal keratinocytes and the dermal infiltrate in cases of DLE; its ligand was expressed within the dermal infiltrate. Over-expression of Fas and Fas-L correlated directly with the extent of apoptosis in DLE and this apoptosis may be directly involved in the pathogenesis of DLE [8].
The aim of this study was to evaluate the role of Fas and Fas-L in one of the ultraviolet-induced tumors, namely BCC and one of the benign ultraviolet-aggravated diseases, namely DLE.
Patients and methods
Patients
The study comprised 40 patients (20 patients with BCC, 20 patients with DLE) and ten control cases. Patients were subjected to full history, especially ultraviolet exposure and previous treatment (excision or radiotherapy in cases of BCC). Dermatological examination included type and site of lesions.
Cases of BCC:
Cases of BCC studied included 12 males (60 %) and eight females (40 %). Their ages ranged from 43 to 84 years with a mean of 64.15 years ± SD 11.34. The mean disease duration was 42.5 months ± SD 35.84. All patients gave a history of sunlight exposure (mostly occupational). Around 50 percent of the patients presented with nodular lesions, the other half presented with ulcerative lesions (rodent ulcer). All the lesions were located on the face, mostly on or around the nose and eyes, or on the cheeks.
Cases of DLE:
Cases of DLE comprised nine males (45 %) and 11 females (55 %). Their ages ranged from 25 to 57 years with a mean of 40.9 years ±SD10.42. The mean disease duration was 48.6 months ±SD50.9. All patients showed photo-exacerbation after sun light exposure. Exposure, is either intermittent or prolonged (mostly occupational). Scalp lesions were associated with cicatricial alopecia in 15 percent of cases. The ten control cases included five males and five females with their ages ranging from 21 to 48 years. They were all healthy with no other skin diseases.
Methods
Specimen collection
Two lesional biopsies were taken from each of the 40 patients. One was preserved in formalin and stained by H&E for histopathological examination to confirm diagnosis and to detect apoptosis. The other one was kept frozen at -80 °C until examined for Fas and Fas-L by PCR. One biopsy was taken from each of the ten control cases (the biopsy was chosen from covered skin in five cases and from exposed skin in five cases).
RNA extraction from skin tissue
RNA was extracted after homogenization using sonicator. Extraction was carried out using QIA amp RNA blood mini kit (Qiagen, Grawley, UK).
RT-PCR
The sequence of the primers used for detection of Fas and Fas-ligand was as follows:
- Fas antigen :
¯5 ACG AAC TTG GAA GGC CTG CAT C 3¯ (Forward)
¯5 TCT GTT CTG CTG TGT CTT GGA C 3¯ (Reverse)
- Fas-ligand:
¯5 CAC CCC AGT CCA CCC CCT GA 3¯ (Forward)
¯5 AGG GGC AGG TTG TTG CAA GA 3¯ (Reverse)
RNA was reversely transcribed using 12.5 µL oligo (dT) 18 primer (final concentration 0.2 µM) and was denatured at 70°C for 2 minutes and then placed in ice for 5 minutes. The RT mix was formed of: First strand buffer, MgCl2 (25mM), RNase inhibitor (40µ/µl), dNTPs (10mM), AMV. The mixture was placed in 42 °C for 1 hour then in 95°C for 5 minutes.
The PCR mix was formed of: 10X Buffer, dNTPs (10 mM), Primer forward, Primer reverse, Taq polymerase, cDNA, in a final volume of 50 µl.
The PCR cycles for Fas: 94 °C for 3 minutes for 1 cycle followed by 35 cycles of 94 °C for 30 seconds, 55 °C for 30 seconds, 72 °C for 45 seconds, then one cycle of 72 °C for 10 minutes.
The PCR condition for Fas-ligand: 94 °C for 3 minutes for 1 cycle, then 30 cycles of 94 °C for 45 seconds, 50 °C for 45 seconds, 72 °C for 1 minute, followed by 72 °C for 7 minutes for 1 cycle
Agarose gel electrophoresis:
The PCR products were electrophoresed on 2 percent agarose gel stained with ethidine bromide giving positive bands at 670 bp for Fas and 480 bp for Fas-L.
Data management and statistical analysis
The data were coded and entered on an IBM compatible computer using the SPSS statistical package version 9.0. The data were summarized using the mean and standard deviation for quantitative data and percent for qualitative data. Statistical tests of significance were used to assess differences between studied groups as indicated. The t-test was used for quantitative data while Chi-square was used for qualitative data [9].
Results
Cases of BCC
The histopathological data as regards the histological type of the tumor, cellular infiltrate, and presence of peritumoral apoptosis are presented in Table 1.
PCR results:
Fas was positive in only one case (5 %). It was negative in the remaining 19 cases (95 %). On the other hand, Fas-L was positive in all (100 %) of the cases studied (Table 2, Fig. 1). The relationships of Fas and Fas-L with the various clinical parameters and histopathological parameters were statistically non-significant. The relationship between PCR results of Fas and Fas-L was also found to be statistically non-significant.
The control cases showed negative results for both Fas and Fas-L except for only one case (case number three) that showed positive Fas and positive Fas-L. The biopsy of this case was taken from a sun-exposed area (dorsum of the hand). The statistical relationship between cases of BCC and control cases was found to be non-significant as regards the Fas results, while Fas-L positive BCC cases were higher than control cases and this difference was statistically significant (p < 0.05) (Table 3). Finally, the effect of sun exposure on the histological type of the tumor, extent of inflammatory infiltrate, and apoptosis was found to be statistically non-significant. This insignificant data may be the result of dividing patients into minimally sun-exposed persons who are mainly housewives or non-working patients, and patients with excessive exposure, who are outdoor workers. This division minimizes the number of patients in each group, which may affect statistical significance.
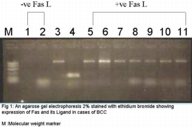 | 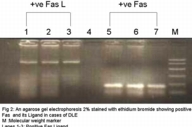 |
| Figure 1 | Figure 2 |
|---|---|
| Agarose gel electrophoresis for BCC (Fig. 1) and for DLE (Fig. 2). Click the figures for the full-size images. | |
Cases of DLE:
Clinical results:
The histopathological data on dermal edema, vascular degeneration, and apoptosis are presented in Table 4.
PCR results:
Fas was found to be positive in 16 cases (80 %) of DLE, and negative in 4 cases (20 %). Fas-L was found to be positive in 18 cases (90 %) and negative in 2 cases (10 %) (Table 5, Fig. 2). Cases 16 and 19 showed evidence of neither Fas nor Fas-L. Cases 15 and 18 showed positive Fas but negative Fas-L. All these four cases showed histopathological evidence of apoptosis in spite of the previously mentioned PCR results of Fas and Fas-L.
The relationship between Fas or Fas L and various clinical and histopathological results was found to be statistically non-significant. A statistical correlation was done between apoptosis-negative-, apoptosis-positive-, Fas-negative-, and Fas-positive-cases without significance (p > 0.05). There was a statistically significant relationship between Fas-positive cases and Fas-L positive cases of DLE (p < 0.005) (Table 6).
The relationship between cases of DLE and control cases showed that, Fas or Fas-L positive cases were higher than Fas positive and Fas-L positive control cases and this difference was statistically significant (p < 0.05) (Table 7).
Discussion
Basal cell carcinoma
Basal cell carcinomas are considered to be the most common human malignancy and UV radiation is the most important physical carcinogen [10]. In this study, apoptosis of peritumoral lymphocytes was detected in 60 percent of cases. In cases of BCC, apoptosis of peritumoral lymphocytes coming to attack the tumor was previously reported [6], and was considered to be one of the mechanisms by which the tumor can escape or bypass the immune system. It was reported that T-lymphocytes adjacent to BCC were found to be undergoing apoptosis, while those not in proximity to tumor cells are viable, which may indicate that tumor cells could be responsible for apoptosis of peritumoral lymphocytes [11]. UVB-radiation plays a role in apoptosis of peritumoral lymphocytes and thus increasing the ability of BCC to escape from immune attack [6], however, in the present study, the effect of sun exposure (UV-radiation) on apoptosis was found to be nonsignificant.
PCR results for Fas in the 20 cases of BCC studied showed that expression of Fas was found to be positive in only 5 percent of cases and negative in the remaining 95 percent of cases. These results were further supported by others [12] who detected no staining for Fas in all nine cases of BCC studied (100 %). The results of the present study would also coincide with the results of another study [5] in which expression of Fas was negative in all BCC cases studied.
In the present study, Fas-L was found to be positive in 100 percent of BCC cases. These results are higher than other reports that indicated Fas-L in 60 percent and 66 percent of BCC cases [5, 13].
The results of both Fas (negative in 95 % of cases) and Fas-L (positive in 100 % of cases) in the present study strongly coincided with studies done by other authors [5, 6] who reported that BCC tumor cells strongly express Fas-L but not the Fas antigen. Also, it was reported that untreated BCC express Fas-L but not the Fas antigen [14].
The relationship between Fas, BCC, and UV radiation was discussed in few recent studies. In one study Fas was not found to be expressed. The same study also noted that with advanced sun exposure and the development of dysplasia and premalignant changes, there was down-regulation of Fas [12]. In addition, ultraviolet-B radiation induces expression of Fas-L by BCC tumor cells [15]. It seems that UVB radiation, by bringing about apoptosis of cytotoxic T-lymphocytes, increases the ability of BCC to escape from immune attack. Because cytotoxic T-lymphocytes express both Fas and Fas-L, and the tumor cells fail to express Fas (as in the 95 % of cases studied in the present study), so there is no interaction between Fas-L on T-cells and tumor cells and thus avoiding apoptosis of tumor cells [6].
The loss of Fas expression in BCC could be attributed to other factors such as p53. It was found to upregulate Fas expression under normal conditions, so a loss-of-function mutation of p53, a well-known event in BCC [16], is usually associated with loss of Fas expression and protection of tumor cells from apoptosis [17, 18, 19]. Accordingly, in the present study, we have to discuss the possible relationships between the presence of peritumoral apoptosis and the PCR results of Fas and Fas-L. Peritumoral lymphocytes are the cells showing apoptosis and the surviving cells are the tumor cells. So, it seems that the killing cells are the tumor cells. Those killing tumor cells carry Fas-L (Fas-L was positive in 100 % of the cases), which induces apoptosis in peritumoral lymphocytes. It was noted that Fas-L-bearing tumor cells can kill peritumoral lymphocytes by a Fas-L-based direct tumor counter-attack or by Fas-L-mediated activation-induced cell death of T lymphocytes [20]. Fas-L on tumor cells can also kill lymphokine-activated killer cells (LAK) coming to attack the tumor [21].
Discoid lupus erythematosus
Apoptosis was detected in 90 percent of cases of DLE studied in this work. Apoptosis is considered one of the important mechanisms of immunological cytotoxicity involved in the pathogenesis of DLE [22]. A pathological relationship between binding of anti-nuclear antibodies and the apoptotic process in the development of cutaneous lupus has been suggested [23]. A recent study reported that there is a correlation between apoptosis and the level of Fas in cases of active lupus [24]. However, this relationship was nonsignificant in the present study.
Two cases of DLE studied in this work (10 %) showed no apoptosis, most probably because of the mild infiltrate. This data was further confirmed by a statistical analysis that detected a significant relationship (p < 0.01) between the extent of the infiltrate and the presence of apoptosis (the more intense the infiltrate, the more evident the apoptosis). Apoptosis was found to be greater in cases of acute cutaneous LE than in cases of DLE, indicating that the amount of apoptosis is increased as the disease is more acute [8]. These data indicate that apoptosis is much less evident in very chronic cases of DLE and this may explain the absence of apoptosis in the two cases of DLE in the present study (reported disease durations of 15 and 4 years).
Fas and Fas-L were significantly higher in cases of DLE studied than in the control cases. Other investigators [8] detected Fas-antigen expression on keratinocytes of epidermis and within the dermal infiltrate in all cases (n = 17) of DLE studied (100 %). In cutaneous lupus, Fas was found to be expressed on lymphocytes as well as keratinocytes [25].
The results of the present study can be compared with a study [8] that detected expression of Fas-L within the dermal infiltrate in all cases of DLE. The authors state that accumulation of Fas-L on inflammatory cells around hair follicles is a hallmark of DLE. Another study [26] detected expression of Fas-L on the surface of auto-reactive B and T lymphocytes in cases of DLE. This Fas-L binds with Fas on the surface of immunoregulatory T-lymphocytes, resulting in apoptosis of those immunoregulatory T-lymphocytes that might control the autoimmune process and hence facilitate escape of those auto-reactive B and T cells from immune surveillance. This will eventually end in sustained release of autoantibodies in cutaneous lupus.
Nagata and Goldstein [27] raised the question does UVB-induction of Fas-L and its subsequent interaction with Fas-bearing immunoregulatory T-cells resulting in their apoptosis, play a role in how UV radiation may predispose to DLE. UV irradiation in a genetically predisposed individual induces a local inflammatory response with the release of cytokines, TNF and INF-γ, up-regulation of ICAM-1 and Fas antigen on the keratinocytes, vasodilatation, accumulation of T-lymphocytes and macrophages in the dermis, and extensive apoptosis in both the dermal and epidermal compartment. The central event at this stage would be the down-regulation of Bcl-2 expression in the basal layer of the epidermis, which makes the keratinocytes susceptible to Fas-dependent apoptosis [8]. The results of the present study are further supported by others [28] who detected Fas and Fas-L in most epidermal keratinocytes and around blood vessels and skin appendages in cases of DLE. These findings again suggest that Fas/Fas-L-mediated apoptosis may be related to the pathogenesis of DLE. It suggests that the expression of Fas in the basal layer of the epidermis and the prominent expression of Fas-L on inflammatory cells around hair follicles may be responsible for the severe inflammation, tissue injury, and hair follicle destruction in DLE. Over-expression of Fas and Fas-L in cases of DLE may correlate directly with the extent of apoptosis [8]. On the other hand, two cases of DLE in this work (16 and 19), showed evidence of neither Fas nor Fas-L expression, and another two cases (15 and 18) showed negative Fas but positive Fas-L expression. All four cases showed histopathological evidence of apoptosis in spite of the previously mentioned PCR results of Fas and Fas-L. This may indicate, in contrast to the previous studies, that Fas/Fas-L interaction may not be responsible alone for the apoptosis observed in cases of DLE and that factors other than Fas and Fas-L (for example TNF, IFNα and ICAM-1) may be responsible, in part, for the apoptosis seen in cases of DLE. This finding was mentioned before [22] who detected Fas antigen in only 26.3 percent of cases of DLE studied suggesting little involvement of Fas /Fas-L system in the keratinocyte apoptosis observed in cases of DLE. They also noted that Fas antigen expressed in cases of DLE might be associated with T-cell activation rather than keratinocyte apoptosis.
Finally, the following hypothesis of the pathogenesis of DLE was suggested [8]: UV irradiation to the skin in a genetically predisposed individual is followed by extensive keratinocyte apoptosis mediated by cytokines, including Fas, Fas-L, TNF, IFN-α and ICAM-1. Down-regulation of Bcl-2 makes the keratinocytes more susceptible to Fas/Fas-L mediated apoptosis.
After revising the results of the previous studies and taking into consideration the results of the present study, it seems that Fas/Fas-L interaction plays a critical role in the apoptosis seen in cases of DLE and hence in the pathogenesis of DLE. Although sunlight is a well-established factor in the induction of BCC and induction and exacerbation of DLE [29], the effect of sun exposure on apoptosis and the expression of Fas and Fas-L was found to be statistically non-significant in the present study.
Accordingly, if we can find drugs that increase Fas expression by tumor cells, it will be possible to induce apoptosis of BCC cells through Fas-Fas ligand interaction and hence tumor regression, also if we find drugs that inhibit Fas or its ligand expression in cases with DLE, we may inhibit apoptosis which plays an important role in the pathogenesis of the disease.
References
1. Yang, X, Rhosravi-Far R, Chang HY, Baltimore D: DAXX, a novel Fas-binding protein that activates JNK and apoptosis, Cell, 89: 1067-1076, 1997.2. Brunner T, Mogil RJ, LaFace D, Yoo NJ, Mahboubi A, Echeverri F: Cell-autonomous Fas (CD95)/Fas-Ligand interactions mediated activation-induced apoptosis in T-cell hybridomas, Nature, 373: 441-444, 1995.
3. Aragane, Y, Kulms D, Metze D, Wilkes G, Poppelmann B, Luger A, Schwarz T: Ultraviolet light induces apoptosis via direct activation of CD95 independently of its ligand CD95L, J Cell Biol, 140: 171-174, 1998.
4. Wehrli p, Viard I, Bullani R, Tschopp J, French LE: Death receptors in cutaneous biology and disease, J Invest Dermatol, 115: 141-148, 2000.
5. Lee SH, Jang JJ, Lee JY, Kim SY, Park WS, Shin MS et al: Fas ligand is expressed in normal skin and some cutaneous malignancies, Br J Dermatol, 139: 186-191, 1998.
6. Gutierrez-Steil S, Wrone-Smith T, Sun X, Kreuger JG, Coven T, Nickoloff BJ: Sunlight-induced basal cell carcinoma tumor cells and ultraviolet-B-irradiated psoriatic plaques express Fas ligand (CD95L), J Clin Invest, 101: 33-39, 1998.
7. Donnelly AM, Halbert AR, Bohr JB: Discoid lupus erythematosus, Aus J Dermatol, 36: 3-12, 1995.
8. Baima B, Sticherling M: Apoptosis in different cutaneous manifestations of lupus erythematosus, Br J Dermatol, 144: 958-966, 2001.
9. Beth DS, Robert GT: Basic and clinical biostatistics. Publisher Appleton and Lange, USA, 1990.
10. Wong DA, Bishop GA, Lowes MA, Cooke B, Barneston R St C, Halliday GM: Cytokines profiles in spontaneously regressing basal cell carcinoma, Br J Dermatol, 143: 91-98, 2000.
11. Wrone-Smith T, Nunez G, Johnson T, Nelson B, Boise LH, Thompson CB et al: Discordant expression of Bcl-x and Bcl-2 by keratinocytes in vitro and psoriatic keratinocytes in vivo, Am J Pathol, 146: 1-10, 1995.
12. Filipowicz E, Adegboyega P, Sanchez RL, Gatalica Z: Expression of CD95 (Fas) in sun-exposed human skin and cutaneous carcinomas, Cancer, 94: 814-819, 2002.
13. Jang TJ: Expression of CD40 and Fas ligand in BowenÕs disease, squamous cell carcinoma and basal cell carcinoma, Yonsei Med J, 43: 304-308, 2002.
14. Beuchner SA, Wernli M, Harry T, Hohn S, Itin P, Erb P: Regression of basal cell carcinoma by intralesional interferon-alpha treatment is mediated by CD95 (Apo-1/Fas)-CD95 ligand-induced suicide, Cancer Immunol Immunother, 100: 2691-2696, 1997.
15. Whiteside TL, Rabinowich H: The role of Fas/Fas-L in immunosuppression induced by human tumors, Cancer Immunol Immunother, 46: 175-184, 1998.
16. Reddy VG, Khanna N, Singh N: Vitamin C augments chemotherapeutic response of cervical carcinoma HeLa cells by stabilizing p53, Biochem Biophys Res Commun, 30: 409, 2001.
17. Kobeyashi T, Ruan S, Jabbur JR, Consoli U, Clodi K, Shiku H et al: Differential p53 phosphorylation and activation of apoptosis-promoting genes Bax and Fas/APO-1 by irradiation and ara-C treatment, Cell Death Differ, 5: 584-591, 1998.
18. Sheikh MS, Fornace AJ Jr: Death and decoy receptors and p53-mediated apoptosis, Leukemia, 14: 1509-1513, 2000.
19. Bartke T, Siegmund D, Peter N, Reichwein M, Henkler F, Scheurich S et al: P53 upregulates cFLIP, inhibits transcription of NF-Kappa B-regulated genes and induces caspase-8-independent cell death of DLD-1 cells, Oncogene, 20: 571-580, 2001.
20. Li JH, Rosen D, Sondel P, Berke G: Immune privilege and Fas-L: two ways to inactivate effector cytotoxic T lymphocytes by Fas-L-expressing cells, Immunology, 105: 263-266, 2002.
21. Yamauchi A, Taga K, Mostowski HS, Bloom ET: Target cell-induced apoptosis of interleukin-2-activated human natural killer cells: roles of cell surface molecules and intracellular events, Blood, 87: 5127-5135, 1996.
22. Fushimi M, Furukawa F, Tokura Y, Itoh T, Shirahama S, Wakita H, et al: Membranous and soluble forms of Fas antigen in cutaneous lupus erythematosus, J Dermatol, 25: 302-308, 1998.
23. Casciola-Rosen LA, Anhalt G, Rosen A: Auto antigens targeted in systemic lupus erythematosus are clustered in two populations of surface structures on apoptotic keratinocytes, J Exp Med, 179: 1317-1330, 1994.
24. Silvestris F, Grinell, D, Tucci M, Cafforio P, Dammacco F: Enhancement of T cell apoptosis correlates with increased serum levels of soluble Fas (CD95/Apo-1) in active lupus, Lupus, 12: 8-14, 2003.
25. Nakajima M, Nakajima A, Kayagaki N, Honda M, Yagita H, Okumura K: Expression of Fas ligand and its receptor in cutaneous lupus and its implication in tissue injury, Clin Immunol Immunopathol, 83: 223-229, 1997.
26. Nagafuchi H, Wakasika L, Takeba Y, Takeno M, Sakane T, Suzuki M:Aberrant expression of Fas ligand on anti-DNA autoantibody secreting B lymphocytes in patients with systemic lupus erythematosus: "immune privilege"-like state of the auto reactive B cells, Clin Exp Rheumatol, 20: 625-631, 2002.
27. Nagata S, Goldstein P: The Fas death factor, Science, 267: 1449-1456, 1995.
28. Zhang M, Zhang Y, Wang L, Li F: Expression of Fas and Fas ligand in lesions of patients with discoid lupus erythematosus, Hua Xi Yi Ke Da Xue Xue Bao, 32: 513-515, 2001(Abstract).
29. Lehmann P, Holzle E, Kind P, Goerz G, Plewig G: Experimental reproduction of skin lesions in lupus erythematosus by UVA and UVB radiation, J Am Acad Dermatol, 22: 181-187,1990.
© 2006 Dermatology Online Journal

