Photodistributed reticulated hyperpigmentation related to diltiazem
Published Web Location
https://doi.org/10.5070/D34580r7rsMain Content
Letter: Photodistributed reticulated hyperpigmentation related to diltiazem
Ane Jaka MD, Arantxa López-Pestaña MD, Anna Tuneu MD, Carmen Lobo MD, María López-Núñez MD, Nerea Ormaechea MD
Dermatology Online Journal 17 (7): 14
Servicio Dermatología, Hospital Donostia, Donostia-San Sebastián, Spain. ajaka@aedv.esAbstract
Diltiazem is a calcium channel blocking agent used for the treatment of hypertension. Cutaneous adverse effects are uncommon. The most frequently reported are itching, urticaria, and maculopapular eruption. A peculiar, cutaneous photodistributed reticulated hyperpigmentation secondary to diltiazem has been recently reported. A 66-year-old white woman with a 2 year history of pruritic hyperpigmented lesions on her face was seen in the clinic. Past medical history was remarkable for hypertension, which had been treated with diltiazem. Physical examination showed slate-gray to brown reticulated hyperpigmentation in the photo-exposed areas of the face and neck. Histological examination revealed interface dermatitis with liquefactive degeneration of the basal layer, necrotic keratinocytes, lymphocytic inflammatory infiltrate, and melanophages in the superficial dermis. A diagnosis of diltiazem-induced hyperpigmentation was established and diltiazem was stopped. Gradual resolution of the hyperpigmentation was observed over the following months. Although diltiazem has been marketed for over 20 years, the first cases of this particular type of reticulated hyperpigmentation were described in 2001. Since then, to our knowledge, only 17 cases have been reported in the literature. In all cases, cutaneous lesions appeared at least 6 months after this treatment had been started. Hyperpigmentation was controlled by means of photoprotection and discontinuation of diltiazem. Diltiazem can produce a characteristic lichenoid dermatitis with reticulated hyperpigmentation on sun-exposed areas.
Introduction
Diltiazem is a calcium antagonist which is widely used in the treatment of hypertension [1]. Cutaneous adverse effects are uncommon [2] and usually occur in the form of pruritus, urticaria, and/or a maculopapular eruption. However, reports of subacute cutaneos lupus erythematosus, Stevens-Johnson syndrome, toxic epidermal necrolysis, vasculitis, and photosensitivity have also been published [3]. A particular type of hyperpigmentation in photoexposed areas secondary to diltiazem has recently been reported. Although this antihypertensive has been used for over 20 years, this particular hyperpigmentation was not described until 2001.
Case synopsis
A 66-year-old woman with a history of hypertension treated with diltiazem (Dinisor retard®) and lisinopril for 5 years is reported. She came to our clinic because of a 2 year history of a cutaneous pruritic eruption on her face and neck, which worsened with sun exposure.
 |  |
| Figure 1a | Figure 1b |
|---|---|
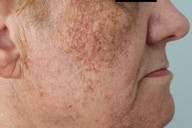
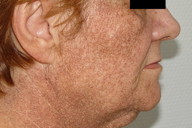
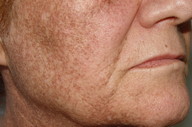
| Figure 1a. Hyperpigmentation in photo-exposed areas of head and neck Figure 1b. Slate-gray brown reticulated hyperpigmentation | |
On examination, a woman with Fitzpatrick type IV skin type exhibited a gray-brown perifollicular and reticulated hyperpigmentation on photoexposed areas (Figures 1a and 1b). The hyperpigmentation spared the centrofacial region, the forehead, and the submental area.
All of the performed laboratory tests were within normal range. Antinuclear antibodies were negative.
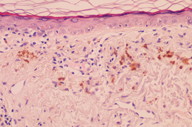
Histopathological examination of a facial biopsy showed epidermal atrophy with flattening of the rete ridges, interface dermatitis with necrotic keratinocytes, discrete perivascular lymphoid infiltrates, and the presence of melanophages in the upper dermis (Figure 2a). The diagnosis of reticulate hyperpigmentation secondary to diltiazem was suspected, and she was advised to replace diltiazem for another antihypertensive drug. Gradual improvement of the hyperpigmentation was observed through the following months (Figure 2b).
The first cases of this particular type of reticulated hyperpigmentation were described in 2001, in 4 African-American women [1]. Since then a total of 17 cases have been reported in the literature. In all these cases the hyperpigmentation is typically grey-brown, has a reticulate pattern, and is limited to photo-exposed areas.
It is more frequently seen in dark-skinned women between the fifth and seventh decades of life; it appears after at least 6 months of drug intake. It has also been reported in men [3, 4] and in light-skinned individuals [5, 6]. It seems remarkable that in all cases the drug involved is the long-acting form of diltiazem [4]. The hyperpigmentation is usually reversible after discontinuation of this drug and total resolution may take many months. The pathogenesis is unknown, but it has been postulated that sun exposure may lead to the formation of free radicals of the drug or its metabolites [5]. Under electronic microscopic examination Scherschun et al [1] observed fully melanized melanosomes compatible with pigmentary incontinence, without deposits of the drug or its metabolites. In a recent study, a range of drug absorption in the spectrum of ultraviolet B was described [4, 7].
Histological examination in this condition shows a lichenoid pattern eruption with necrotic keratinocytes, pigmentary incontinence, and lymphocytic infiltration [8]. The drugs that are most frequently associated with lichenoid eruption are gold salts, antimalarials, penicillamine, thiazide diuretics, beta blockers, inhibitors of angiotensin converting enzyme, and non-steroidal anti-inflammatory drugs. Some authors suggest that the long-acting form of diltiazem should be included as a possible photodistributed lichenoid eruption inducing drug [7].
Prevention of this eruption may be possible with the use of appropriate photoprotection, which should be initiated in all patients starting antihypertensive treatment with diltiazem [5]. Once hyperpigmentation has been established, treatment should also include the replacement of the drug.
References
1. Scherschun L, Lee MW, Lim HW. Diltiazem-associated phototdistributed hyperpigmentation: a review of 4 cases. Arch Dermatol. 2001; 137(2):179-182. [PubMed]2. Chawla A, Goyal S. Diltiazem-induced hyperpigmentation in an African American woman. J Am Acad Dermatol. 2002; 46(3):468-469. [PubMed]
3. Boyer M, Katta R, Markus R. Diltiazem-induced photodistributed hyperpigmentation. Dermatol Online J. 2003; 9(5):10. [PubMed]
4. Hanson M, Petronic-Rosic V. Reticulated phototoxic eruption in a patient on long-term Diltiazem therapy. J Drugs Dermatol. 2008; 7(8):792-3. [PubMed]
5. Saladi RN, Cohen SR, Phelps RG, et al. Diltiazem induces severe photodistributed hyperpigmentation: case series, histoimmunopathology, management, and review of the literature. Arch Dermatol. 2006; 142(2):206-210. [PubMed]
6. Berendzen SM, Carey JD, Smith EB. Diltiazem-associated photodistributed hyperpigmentation in an elderly Hispanic female. Int J Dermatol. 2006; 45(12):1450-2. [PubMed]
7. Desai N, Alexis AF, DeLeo VA. Facial hyperpigmentation caused by diltiazem hydrochloride. Cutis. 2010; 86(2):82-4. [PubMed]
8. Kubo Y, Fukumoto D, Ishigami T, Hida Y, Arase S. Diltiazem-associated photodistributed hyperpigmentation: report of two Japanese cases and published work review. J Dermatol. 2010; 37(9):807-11. [PubMed]
© 2011 Dermatology Online Journal