Atopic dermatitis and systemic autoimmune diseases: A descriptive cross sectional study
Published Web Location
https://doi.org/10.5070/D33sf31124Main Content
Atopic dermatitis and systemic autoimmune diseases: A descriptive cross-sectional study
Vida Feizy, Arefeh Ghobadi
Dermatology Online Journal 12 (3): 3
Department of dermatology, Tehran university of medical sciences (TUMS). v.feizy@doctor.comAbstract
Atopic dermatitis (AD) has a Th2 (T-helper 2) immune-reactivity pattern. However, the majority of systemic autoimmune diseases, such as rheumatoid arthritis, multiple sclerosis, and insulin dependent diabetes mellitus show a Th1 (T-helper 1) reactivity pattern. From this, one may hypothesize that AD and the Th1 autoimmune diseases could be inversely associated and AD may be more common in the minority of autoimmune diseases with a Th2 overactivity pattern such as systemic lupus erythematosus. A cross sectional study was designed. Our patients were enrolled from a general university hospital (all systemic autoimmune patients in every medical ward based on definite diagnoses in their medical records). Information on atopic dermatitis was obtained by questionnaires and physical examination by a dermatologist. A total of 63 patients were studied; 17.5 percent of cases had atopic dermatitis in the past or present. There were 31 patients 49.2 %) who carried a diagnosis known to be associated with Th1 reaction, and 21 patients (33.3 %) who had a disease associated with Th2-type reactivity. In 11 patients (17.5 %) the T-cell reaction type was not definitively classified. The relative frequency of AD was 9.7 percent (3 of 31 cases) in Th1-related autoimmune diseases, 28.6 percent (6 of 21 cases) in Th2-related autoimmune diseases and 18.2 percent (2 of 11 cases) in the unclassified category, a difference not statistically significant. Although the power of this study is not high enough to show a statistical significance, AD seems to be uncommon in patients with autoimmune diseases associated with Th1 overactivity.
Introduction
Atopic dermatitis (AD) is an itchy, chronic or chronically relapsing, inflammatory skin condition. The eruption is characterized by itchy papules that become excoriated and lichenified [1]. Immune dysregulation is an important factor in the creation of this condition. A defining characteristic of the atopic immune system is the capacity to generate elevated IGE antibodies and the key disturbance to immune regulation is in the differentiation pathway of T-helper 0 (Th0) cells. These precursor cells are induced to differentiate into T-helper 2 (Th2) cells that typically produce IL-4, IL-5, and IL-13. Th2 cells control the synthesis of IGE [1]. Th1 and Th2 cells have been found to be mutually antagonistic, leading to either Th1- or Th2-dominated responses upon immunization. Because in chronic inflammatory autoimmune diseases such as insulin dependent diabetes mellitus (IDDM), multiple sclerosis (MS) and rheumatoid arthritis (RA) Th1 cells are pathogenic and Th2 cells are protective, one might expect a negative association between AD with Th2 phenotype and diseases with Th1 phenotype [2]. In systemic lupus erythematosus (SLE) and ulcerative colitis (UC), a Th2 response predominates [3, 4, 5], therefore one might expect a positive association between these diseases and AD in this population. We compared the frequency of AD in these two groups of systemic autoimmune diseases. We used the UK diagnostic criteria for AD which is the most suitable one for determining this disease in population based studies.
Methods
A descriptive cross sectional study was designed. Our cases were enrolled from a university hospital (all systemic autoimmune patients in every medical ward), based on their definite diagnosis in the medical records. The list of expected systemic autoimmune diseases is as follows in Table 1 [6].
|
Among these diseases RA, IDDM, and MS are Th1-related [2]. SLE and UC are Th2-related [3, 4, 5]. The other diseases listed are unclassified. The diagnosis of AD was determined for these patients using a questionnaire based on UK diagnostic criteria [7] and physical examination by a dermatologist (p = 0.05).
Results
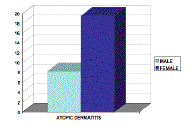
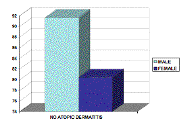
Of the 63 cases collected, 12 (19 %) were male, and 51 (81 %) were female. The mean age of our patients was 34.8 with a range between 13 and 70 (SD = 14.87). The patients with Th1-related diseases had an age range of 15-70 years with a mean of 39.48 (SD = 15.94). Patients with Th2-related diseases had an age range between 13-42 years with a mean of 24.6 years (SD = 7.85). Unclassified patients were between 23 and 64 years with a mean age of 41 years (SD = 12.68). Atopic dermatitis, past or present, was identified in 17.5 percent of patients studied. The relative frequency of AD was 8.3 percent among men and 19.6 percent among women, a difference not statistically significant (p > 0.05) (Fig. 1).
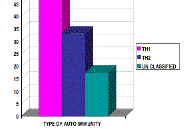
Systemic autoimmune diseases observed in our patients were as follows: 24 cases of RA (38.1 %), 20 cases of SLE (33.1 %), 6 cases of IDDM (9.5 %), 6 cases of scleroderma (9.5 %), 2 cases of myasthenia gravis (3.2 %), 2 cases of polymyositis (3.2 %), 1 case of UC (1.6 %), 1 case of MS (1.6 %), and 1 case of dermatomyositis (1.6 %). Therefore, 49.2 percent (31 cases) of our autoimmune disease patients exhibited diseases of Th1 reactivity and 33.3 percent (21 cases) Th2 reactivity. Eleven patients (17.5 %) suffered from unclassified autoimmune diseases.
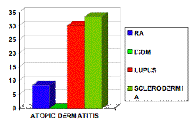
Atopic dermatitis was found in 12.5 percent (3 of 24) of RA patients, 30 percent (6 of 20) of SLE patients, 33.3 percent (2 of 6) of scleroderma patients, none (0 of 6) of IDDM cases, and 17.5 percent of all cases (Fig. 3). The relative frequency of AD was 9.7 percent (3 of 31) in Th1-related diseases, 28.6 percent (6 of 21) in Th2-related diseases and 18.2 percent (2 of 11) in unclassified ones (p > 0.05) (Fig. 4).
Conclusion
Most of the known autoimmune diseases are due to Th1 reactivity [2]; our finding of 49.2 percent Th1 versus 33.3 percent Th2 is consistent with this. Atopic patients are known to have hyper-reactivity of the Th2 immune mechanism [1].
Few studies have investigated the association between autoimmune diseases and those AD, which is mainly Th2-related. The purpose of this report is to help to determine if there is an association between systemic autoimmune diseases and atopic dermatitis. There is often a reciprocal relationship between Th1 and Th2 immune responses, which suggests that atopic dermatitis (Th2 phenotype) and RA, MS, and IDDM (Th1 phenotype) are unlikely to coexist in the same individual. There may be also a positive association between AD and autoimmune diseases with Th2 phenotype (SLE and UC), which comprise a minority of systemic autoimmune diseases. Th1 and Th2 cells can antagonize each other either by blocking the generation of the other cell type, or by opposing effector functions. For instance, the presence of high concentrations of IL-4 or IL-6 blocks the generation of Th1 cells from naïve T cells, even in the presence of IL-12. At the level of effector functions, IFN-gamma secreted by Th1 cells block the proliferation of Th2 cells. Analogously, IL-10, which is produced in large amounts by Th2 cells, inhibits macrophage functions such as secretion of IL-12. IL-10 also antagonizes the up-regulation of major histocompatibility complex (MHC) molecules stimulated by IFN-gamma [2].
According to previous studies carried out in our country, the prevalence of AD in 16-25 year-old men of Sanandaj city in Iran was 14.5 percent [8]. Another study, performed in Bushehr city in Iran, reported a prevalence of 7.9 percent among 13-14-year-old school children [9]. In that case, the finding of 28.6 percent prevalence of AD among patients suffering from Th2-related autoimmune diseases is clinically important. We find that there is a lower frequency of AD in diseases of Th1 (9.7 %) although it was not statistically significant due to the small sample size.
According to the results of a Danish study, the incidence of AD up to age 15 is 13.1 percent among children with IDDM and 19.8 percent in nondiabetic children (p < 0.0001) [10].
Furthermore, although AD is almost exclusively characterized by Th2-like reactions in acute forms of AD, Th1-like reactions are documented in forms of chronic eczema [11, 12]. This may relate to the finding of 9.7 percent prevalence for AD among the Th1 autoimmune group. Other factors are also certainly involved. Inability to categorize all of autoimmune diseases into Th1- or Th2-dominated groups is a problem in our study, because diseases such as scleroderma and polymyositis are unclassified.
We cannot rule out bias due to recall differences and cannot assess the direction or magnitude of any such bias. The magnitude of recall bias probably partially depends on the age of patients in the both groups of the study.
Larger studies comparing AD in autoimmune Th1 and Th2 autoimmune diseases are needed along with studies of differences in serum IgE and cytokine profiles among patients, ideally with prospective cohort designs.
AD Atopic dermatitis IDDM Insulin dependent diabetes mellitus MHC Major histocompatibility complex MS Multiple sclerosis PAN Poly arthritis nodosa PBC Primary biliary cirrhosis RA Rheumatoid arthritis SLE Systemic lupus erythematosus Th0 T helper 0 Th1 T helper 1 Th2 T helper 2 |
Aknowledgment: We would like to thank Dr. Fatemeh Esfahani for her kind help in the analysis of data.
References
1. Friedman PS, Holden CA. Atopic dermatitis in Rook's Text Book of Dermatology.7th edition , ed. Burns T, Breathnach S, Cox N,MA:Blackwell; 20042. Lafaille JJ. The role of helper T cell subsets in autoimmune diseases. Cytokine Growth Factor Rev. 1998 Jun;9(2):139-51. PubMed
3. Funauchi M, Ikoma S, Enomoto H, Horiachi A. Decreased Th1-like and increased Th2-like cells in systemic lupus erythematosus. Scand J Rheumatol. 1997;27(3)219-224.PubMed
4. Horwitz DA, Gray JD, Behrendsen SC, Kubin M, Rengaraju M, Ohtsuka K, Trinchieri G. Decreased production of IL-12 and other TH1-type cytokines in patient with recent onset systemic lupus erythematosus. Arthritis Rheum. 1998;41(5)838-844. PubMed
5. Friedman S, Blumberg RS. Inflammatory bowel disease in Harrison`s principles of internal medicine.Volume 3,15th edition, ed. Braunwald E, Fauci AS, Kasper DL, Hauser SL, Longo D L, Jameson J.L, N.Y. 838-844, 2001
6. Cotran RS, Kumar V, Collins T. Immune system in Robbins`pathologic basis of disease.Volume 1, 6 th edition, ed. Cotran RS, Kumar V, Collins T, Philadelphia, 1679-1681, 1999
7. Williams H. Disease definition and measures of disease frequency.J Am Acad Dermatol.2001; 5(1)833-36.PubMed
8. Rahmani MR, Zarei M. Effect of socio-economic status on level of atopic disease in 16-25 years old men in Sanandaj city. Journal of Babol university of medical science (published in Iran) 2004;2(6)30-34
9. Hatami G, Amir Azodi E, Najori A. Prevalence of Asthma and Asthma related symptoms among 13-14 years old school children in Bushehr. ISSAC Iranian south medical journal (published in Iran). 2002;2(5)167-175
10. Olesen AB, Jull S, Birkebaek N, Thestrup-pedersen K. Association between atopic dermatitis and insulin dependent diabetes mellitus:a case control study. Lancet.2001;(357)1749-752.PubMed
11. Grewe M, Gyfko K, Schopf E, Knutmann J. Lesional expression of interferon-gamma in atopic eczema. Lancet. 1994 ;(343)25-26.PubMed
12. Thepen T, Langeveld-Wildschut EG, Bihari IC. Biphasic response against aeroallergen in atopic dermatitis showing a switch from initial TH2 response to a TH-1 response in situ:an immunocytochemical study. J Allergy Clin Immunol. 1996; (97)828-837.PubMed
© 2006 Dermatology Online Journal