Successful long-term management of refractory cutaneous and upper airway sarcoidosis with periodic infliximab infusion
Published Web Location
https://doi.org/10.5070/D33n87w6h3Main Content
Successful long-term management of refractory cutaneous and upper airway sarcoidosis with periodic infliximab infusion
Ted Rosen MD, Christy Doherty MD
Dermatology Online Journal 13 (3): 14
Departments of Dermatology, Baylor College of Medicine and Veterans Affairs Medical Center Houston, TexasAbstract
A clinically refractory case of cutaneous sarcoidosis was successfully treated with infliximab infusion therapy at a dose of 5mg/kg. Skin lesions cleared dramatically and remain resolved during a 3½ year follow-up, the longest follow-up reported to date. Ongoing biological drug therapy has been administered at a frequency of every 8-10 weeks. Despite cost and safety concerns, infliximab should be considered in cases of cutaneous sarcoidosis refractory to standard treatment protocols.
Cutaneous sarcoidosis can be either relatively easy or moderately difficult to manage, independent of other manifestations of disease activity. We present herein an essentially refractory case of disfiguring cutaneous and upper airway sarcoidosis that demonstrated a gratifying response to regular periodic infusions of the chimeric anti-tumor-necrosis-factor-α (anti-TNF-α) monoclonal antibody, infliximab.
Clinical synopsis
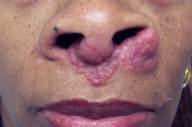 |
| Figure 1 |
|---|
| Figure 1: Pre-infliximab treatment cutaneous sarcoidosis |
A 52-year-old woman presented with gradually worsening skin lesions of approximately 5-years duration. Physical examination disclosed multiple violaceous facial papules and nodules, consistent with the lupus pernio subtype of cutaneous sarcoidosis. Lesions were especially notable on and near the nares. Similar nodular lesions along the nasal septum were protruding into the nasal passage and causing some difficulty breathing. Prior skin biopsies demonstrated non-caseating granulomata, consistent with the diagnosis of sarcoidosis. The patient had responded only minimally to prior oral hydroxychloroquine treatment (200mg twice daily), and she was therefore seeking a second opinion.
Repeat skin biopsy was again consistent with sarcoidosis. Laboratory examination, including CBC, liver and renal function studies, serum calcium, serum protein electrophoresis, and angiotensin-converting-enzyme level were normal. Chest radiographs revealed bilateral hilar adenopathy and a suggestion of parenchymal fibrosis, although pulmonary functions were normal.
The patient was initially treated with ultrapotent topical and intralesional steroids, with no improvement. Prednisone (up to 60mg daily) given as monotherapy and subsequently in combination with both chloroquine (500mg daily) and methotrexate (30mg weekly) failed to improve skin lesions. Pentoxifylline, 400mg three times daily as an adjunctive treatment, also failed to alter the clinical course, as did both high dose tetracycline (2 grams daily) and minocycline (200 mg daily). Despite 6 months of therapy as detailed above, cutaneous lesions actually worsened (Fig. 1). The patient was both distressed and frustrated by the failure of all attempted therapeutic agents.
After extensive counsel, the patient agreed to undergo off-label anti-TNF-α therapy with intravenous infliximab infusion at a dose of 5mg/kg/session. A pre-treatment screening panel was negative (PPD, CBC, liver function studies). Initial treatment was given at weeks 0, 2 and 6, the standard method of administration for FDA-approved clinical indications. Lesions rapidly and dramatically reduced in intensity after the first three infusions. (Fig. 2). Following two additional infusions (weeks 14 and 22), almost no clinical evidence of cutaneous or intranasal disease remained. Four additional infusions were given at 8 week intervals, and then frequency of infusion was reduced to every 8-10 weeks for an additional two and a half years. The patient remained in a clinical remission at the 3½ year follow-up visit (Fig. 3), and continues to receive ongoing infliximab uneventfully.
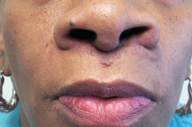 | 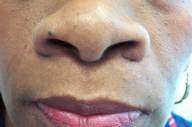 |
| Figure 2 | Figure 3 |
|---|---|
| Figure 2: Lesions after three infliximab infusions Figure 3: Appearance at three and a half years of therapy | |
Discussion
Treatment of sarcoidosis is often complicated by variable and unpredictable response to therapy. For cutaneous sarcoidosis, standard therapy has traditionally included topical, intralesional, and systemic steroids, antimalarial drugs, methotrexate, and combinations of the aforementioned agents [1]. However, cases of sarcoidosis refractory to standard treatments and the potential toxicities associated with these agents have prompted innovative treatment options, including tetracycline antibiotics, isotretinoin, anti-TNF-α agents, thalidomide, allopurinol, cyclosporin-A, chlorambucil, leflunomide, and melatonin [1].
After our patient failed treatment with both standard therapy and pentoxifylline, infliximab was considered due to reported cases of refractory sarcoidosis responding to this agent [2, 3, 4, 5, 6, 7]. Release of TNF-α has been found to be increased in patients with sarcoidosis, and TNF-α is a crucial pro-inflammatory cytokine necessary for the formation and maintenance of granulomas, the characteristic component of sarcoidosis [8]. The production of TNF-α in a sustained manner leads to continued recruitment of inflammatory cells into granulomas, tissue consolidation and functional impairment.
Infliximab is a chimeric monoclonal anti-TNF antibody that binds to and thereby directly inactivates this critical cytokine [9]. Additionally, infliximab has been shown to bind membrane-bound TNF-α in vitro, resulting in cell lysis of TNF-α-expressing cells via antibody-dependent cellular toxicity and complement-dependent cytotoxicity [10]. In essence, therefore, the pathological granulomata not only fail to maintain but actually break down.
Baughman and Lower report the improvement of lupus pernio in two patients 16 weeks after initiation of infliximab treatment [2]. Meyerle and Shorr later reported the complete resolution of cutaneous disease and stabilization of pulmonary symptoms after infliximab initiation [3]. The improvement of progressive cutaneous sarcoidosis was reported by Mallbris et al. in a patient after four treatments with infliximab [4]. Methotrexate was begun at 7.5 mg/wk to suppress the potential development of antibodies to infliximab 2 weeks prior to infliximab initiation in this patient. Roberts et al. report the dramatic improvement of oculocutaneous sarcoidosis with resolution of periocular cutaneous lesions after 1 month of infliximab therapy [5]. Haley et al. report the successful treatment of lupus pernio, pulmonary sarcoidosis, and bone cysts with 5mg/kg of infliximab given concomitantly with a slow prednisone taper [6]. Recently, Heffernan and Anadkat reported 90 percent clearance of cutaneous lesions in a patient by week 6 of infliximab therapy [7]. In a retrospective review of ten patients who received infliximab for sarcoidosis, all demonstrated objective evidence of improvement over treatment periods of 6 weeks to 2½ years [11]. Five of six patients receiving corticosteroids prior to infliximab treatment were able to reduce steroid dosage. Of five patients with lupus pernio and one patient with non-lupus pernio cutaneous sarcoidosis, all experienced significant improvement of lesions. Followup provided for several patients with cutaneous disease included one whose lupus pernio lesions had not recurred following discontinuation of infliximab (no time period provided) and one patient who discontinued infliximab after 1 year because of repeated infusion reactions consisting of flushing, hypotension, and chest pain.
Although successful use of infliximab is reported in the literature, there is little discussion regarding long-term response to this modality. We report that our patient continues to be in clinical remission well over 3 years following infliximab initiation. She receives 5 mg/kg of infliximab every 8-10 weeks and shows no signs of either cutaneous or upper airway disease. We doubt that she is cured and prefer to consider this a long-term maintenance therapy (as it is viewed for inflammatory bowel disease).
Infliximab is an immunosuppressive agent, and patients receiving this therapy may be at an increased risk of developing lymphoma and viral or granulomatous infections including tuberculosis, histoplasmosis, coccidioidomycosis, cryptococcal infection and blasotmycosis [12]. Of particular concern is infliximab's association with an increased reactivation rate of tuberculosis. Sarcoidosis is a diagnosis of exclusion, and one must be careful to avoid mistaking tuberculosis as sarcoidosis or mislabeling the signs and symptoms of active tuberculosis as worsening sarcoidosis.
Large, randomized controlled trials are needed before the definite efficacy and safety of infliximab in the various subtypes of sarcoidosis can be determined. However, reports of refractory cutaneous sarcoidosis treated successfully with infliximab support the utility of this agent in cases of otherwise resistant disease. Additionally, our experience with a patient who achieved a long-term, consistent response lends hope that infliximab may provide some patients with extended disease-free periods.
References
1. Badgwell C, Rosen T. Cutaneous sarcoidosis therapy updated. J Am Acad Dermatol 2007;56(1):69-83. PubMed2. Baughman RP, Lower EE. Infliximab for refractory sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis 2001;18(1):70-74. PubMed
3. Meyerle JH, Shorr A. The use of infliximab in cutaneous sarcoidosis. J Drugs Dermatol 2003;2(4):413-414. PubMed
4. Mallbris L, Ljungberg A, Hedblad MA, Larsson P, Stahle-Backdahl M. Progressive cutaneous sarcoidosis responding to anti-tumor necrosis factor-alpha therapy. J Am Acad Dermatol 2003;48(2):290-293. PubMed
5. Roberts SD, Wilkes DS, Burgett RA, Knox KS. Refractory sarcoidosis responding to infliximab. Chest 2003;124(5):2028-2031. PubMed
6. Haley H, Cantrell W, Smith K. Infliximab therapy for sarcoidosis (lupus pernio). Br J Dermatol 2004;150(1):146-149. PubMed
7. Heffernan MP, Anadkat MJ. Recalcitrant cutaneous sarcoidosis responding to infliximab. Arch Dermatol 2005;141(7):910-911. PubMed
8. Baughman RP, Iannuzzi M. Tumor necrosis factor in sarcoidosis and its potential for targeted therapy. BioDrugs 2003;17(6):425-431. PubMed
9. Scallon B, Cai A, Solowski N, et al. Binding and functional comparisons of two types of tumor necrosis factor antagonists. J Pharm Exp Ther 2002;301(2):418-426. PubMed
10. Scallon BJ, Moore MA, Trinh H, Knight DM, Ghrayeb J. Chimeric anti-TNF monoclonal antibody cA2 binds recombinant transmembrane TNF-alpha and activates immune effector functions. Cytokine 1995;7(3):251-259. PubMed
11. Doty JD, Mazur JE, Judson MA. Treatment of sarcoidosis with infliximab. Chest 2005;127(3):1064-1071. PubMed
12. Furst DE, Wallis R, Broder M, Beenhouwer DO. Tumor necrosis factor antagonists: Different kinetics and/or mechanisms of action may explain differences in the risk for developing granulomatous infection. Semin Arthritis Rheum. 2006;36(3):159-67. PubMed
© 2007 Dermatology Online Journal

