fungemia in an immunocompromised patient
Published Web Location
https://doi.org/10.5070/D33kt2x8r2Main Content
Candida krusei fungemia in an immunocompromised patient
Jonathan L Hager MD, Mohsin R Mir MD, Sylvia Hsu MD
Dermatology Online Journal 16 (4): 5
Department of Dermatology, Baylor College of Medicine, Houston, Texas. shsu@bcm.eduAbstract
Candida krusei is an emerging fungal pathogen found primarily in immunocompromised patients. Intrinsic resistance to fluconazole and decreasing susceptibility to other anti-fungal agents are problematic. When colonization occurs, dissemination may follow rapidly. We present a case of a patient with acute lymphoblastic leukemia who, despite being treated prophylactically with fluconazole, developed disseminated C. krusei.
Introduction
Candida krusei is an emerging fungal pathogen found primarily in immunocompromised patients. Intrinsic resistance to fluconazole and decreasing susceptibility to other anti-fungal agents are problematic. When colonization occurs, dissemination may follow rapidly. We present a case of a patient with acute lymphoblastic leukemia who, despite being treated prophylactically with fluconazole, developed disseminated C. krusei.
Case report
A 23-year-old Hispanic man with a 2-year history of pre-B cell acute lymphoblastic leukemia treated with multiple rounds of chemotherapy presented with fever, weakness, and dyspnea on exertion for 3 days. Laboratory studies showed a white blood cell count of 200/mL, platelet count of 1000/mL, and hemoglobin of 3.9 g/dL. He was diagnosed with neutropenic fever and pan-cultures were drawn. Empiric intravenous vancomycin, cefepime, gentamycin, acyclovir, and fluconazole were administered. Subsequently, blood cultures grew extended spectrum β-lactam-susceptible E. coli. All antimicrobials except vancomycin were discontinued and meropenem was added. After 3 days, the patient was afebrile and repeat blood cultures taken 6 days after admission were negative.
 |  |
| Figure 1 | Figure 2 |
|---|---|
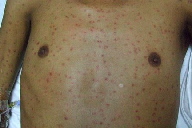
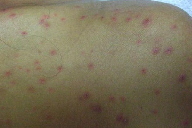
| Figure 1. Diffuse papular eruption was noted on physical examination. Figure 2. Crusted papules on the left shoulder | |
At that time, the patient underwent repeat chemotherapy treatment. After completing 5 days of hyper-CVAD (cyclophosphamide, vincristine, doxorubicin, and dexamethasone) with asparaginase, the patient again developed neutropenic fever. He remained febrile despite broad-spectrum antimicrobials and developed a generalized eruption. Dermatology consultation was obtained for possible drug eruption versus infection. Skin examination revealed a diffuse eruption of erythematous, necrotic papules, some with crusting (Figures 1 and 2).
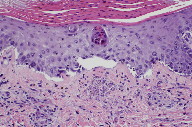
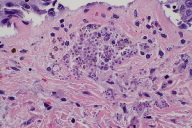
A 6 mm punch biopsy specimen was bisected for histological examination and tissue culture. Biopsy revealed a parakeratotic epidermis with an inflammatory infiltrate in the dermis (Figure 3). Aggregates of spores consistent with Candida infection were noted in the upper dermis (Figure 4). Blood and tissue cultures revealed the presence of Candida krusei. Despite the addition of amphotericin B and micafungin, the patient’s condition worsened. He developed hypotension, anasarca, scleral icterus, and a pericardial effusion. Subsequently, the patient elected to be discharged to home hospice and expired shortly thereafter.
Discussion
The incidence of Candida krusei infections has increased over the last few decades making it an important pathogen in the differential diagnosis of infection in immunocompromised patients [1]. Candida krusei infections typically arise in patients in whom prophylactic treatment with antifungal agents has selected for resistant species of fungi. The incidence of C. krusei has been shown to be up to 7 times greater in neutropenic or bone marrow transplant patients who are receiving fluconazole prophylaxis than those not receiving prophylaxis [2]. As with selection of Clostridium difficile via broad-spectrum antibiotics, it is hypothesized that the destruction of bacterial and fungal colonization may allow resistant pathogens to infect the individual [3, 4]. Although there is a strong association with fluconazole prophylaxis, nosocomial settings, and neutropenia, cases of disseminated C. krusei have been described in immunocompetent patients acquiring the infection in a community setting [3]. As opposed to C. albicans, which most commonly infects the bloodstream from a catheter-related source, the origin of C. krusei fungemia is usually unknown and most often secondary to colonization. Colonization occurs most frequently in the gastrointestinal system, followed by the respiratory and urinary systems. The frequent gastrointestinal colonization may be due to extensive use of antibiotic prophylaxis [5].
The increased incidence of disseminated C. krusei in patients receiving fluconazole prophylaxis is not a contraindication to the use of the drug. It is widely implemented for fungal prophylaxis in immunocompromised patients and has been shown to reduce overall fungal-associated mortality, incidence of graft-versus-host disease, and cyclophosphamide toxicity [6]. Studies have shown that fluconazole 400 mg/day is effective in lowering morbidity and mortality in patients who have received allogeneic stem cell transplants. However, no overall mortality benefit has been shown with fungal prophylaxis in patients with acute leukemia [7].
Candida krusei infection may present in a variety of manners. Mild local infection may produce skin lesions nearly identical to those of C. albicans. In disseminated disease, patients often present with fever, papular eruption, and multi-organ failure. There is a high rate of mortality, particularly in patients with leukemia who develop C. krusei fungemia. The majority of studies show a significantly higher rate of mortality associated with C. krusei fungemia compared to that of C. albicans fungemia [1, 3]. Particularly important predisposing factors for C. krusei fungemia are acute leukemia and neutropenia. Poor response to treatment has been associated with persistent neutropenia, disseminated infection, septic shock, other concomitant fungal infections, and immunosuppressive therapy [1].
Candida krusei resistance to fluconazole is inherent. Orozco et al. demonstrated that the resistance is due to decreased susceptibility of 14α-demethylase to the inhibitory effects of fluconazole rather than decreased drug accumulation [8]. Flucytosine was formerly very effective against this pathogen, but recent studies have shown markedly diminished susceptibility to this agent. Echinocandins and amphotericin B are still employed to treat, but there are some reports that indicate that resistance to these anti-fungal agents is emerging [1, 9]. In neutropenic patients with fungemia, current treatment guidelines recommend that voriconazole, an echinocandin, or amphotericin B be utilized until susceptibilities are determined [10].
Diagnosis of disseminated C. krusei is made by blood culture and microscopic examination of the fungal organisms. Diagnostic criteria include absence of germ tube production, fermentation and assimilation of only glucose, production of characteristic hyphae and blastospores, and weak or absent urease activity [5]. To exclude Candida lambica, it may be necessary to examine colony growth at 42°C; C. lambica will not grow at this temperature in contrast to C. krusei. [5, 8].
In conclusion, disseminated C. krusei must be considered in any immunocompromised patient with fever, skin lesions, or organ failure. Because of the aggressive nature of this pathogen and its inherent resistance to antifungal medications, prompt diagnosis and treatment may increase the chance for a favorable outcome.
References
1. Abbas J, Bodey GP, Hanna HA, Mardani M, Girgawy E, Abi-Said D, et al. Candida krusei fungemia. An escalating serious infection in immunocompromised patients. Arch Intern Med 2000;160:2659-64. [PubMed]2. Wingard J. R., W. G. Merz, M. G. Rinaldi, T. R. Johnson, J. E. Karp, and R. Saral. 1991. Increase in Candida krusei infection among patients with bone marrow transplantation and neutropenia treated prophylactically with fluconazole. N. Engl. J. Med. 325:1274-1277. [PubMed]
3. Munoz P, Sanchez-Somolinus M, Alcala L, Rodriguez-Creixems M, Pelaez T, Bouza E. 2005. Candida krusei fungemia: antifungal susceptibility and clinical presentation of an uncommon entity during 15 years in a single general hospital. J. Antimicrobial Chemother. 55:188-193. [PubMed]
4. Hautala T., I. Ikaheimo, H. Husu, et al. A cluster of Candida krusei infections in a haematological unit. BMC infectious Diseases 2007; 7:97. [PubMed]
5. Merz WG, Karp JE, Schron D, Saral R. Increased incidence of fungemia caused by Candida krusei. J Clin Microbiol. 1986;24:581-584. [PubMed]
6. Safdar A., F. van Rhee, J. P. Henslee, et al. Candida glabrata and Candida krusei fungemia after high-risk allogeneic marrow transplantation: no adverse effect of low-dose fluconazole prophylaxis on incidence and outcome. Bone Marrow Transplantation. 2001; 873-878, Vol 28, No 9. [PubMed]
7. Cornely O. A., A. Böhme, D. Buchheidt, et al. Primary prophylaxis of invasive fungal infections in patients with hematologic malignancies. Recommendations of the Infectious Diseases Working Party of the German Society for Haematology and Oncology. Haematologica, Vol 94, Issue 1, 113-122. [PubMed]
8. Orozco AS, Higginbotham LM, Hitchcock CA, et al. Mechanism of Fluconazole Resistance in Candida krusei. Antimicrob Agents Chemother. 1998 Oct;42(10):2645-9. [PubMed]
9. Pfaller, M. A., D. J. Diekema, D. L. Gibbs, et al. Candida krusei, a Multidrug-Resistant Opportunistic Fungal Pathogen: Geographic and Temporal Trends from the ARTEMIS DISK Antifungal Surveillance Program, 2001 to 2005. Journal of Clinical Microbiology, February 2008, p. 515-521, Vol. 46, No. 2. [PubMed]
10. Pappas, P. G., C. A. Kauffman, D. Andes, et al. Clinical practice guidelines for the management of Candadiasis: 2009 Update by the Infectious Diseases Society of America. Clinical Infectious Diseases. 2009; 48:503-35. [PubMed]
© 2010 Dermatology Online Journal