Diltiazem-induced photodistributed hyperpigmentation
Published Web Location
https://doi.org/10.5070/D33c97j4z5Main Content
Diltiazem-induced photodistributed hyperpigmentation
Molly Boyer, Rajani Katta MD, and Ramsey Markus MD
Dermatology Online Journal 9 (5): 10
Abstract
Diltiazem is a calcium-channel antagonist commonly prescribed in the treatment of cardiovascular disease. Although an extensive spectrum of cutaneous reactions to diltiazem has been described, only two published reports of hyperpigmentation induced by diltiazem are known. We report the cases of a 71-year-old black male and a 49-year-old Hispanic male, who both presented with characteristic hyperpigmentation on sun-exposed areas after taking an extended-release form of diltiazem hydrochloride (Tiazac™).
Introduction
Diltiazem hydrochloride, a benzothiazepine calcium-channel blocker, is an infrequent cause of a wide range of skin reactions, varying from the more common exanthematous rashes to severe cutaneous reactions such as Stevens-Johnson syndrome and toxic epidermal necrolysis [1, 2, 3]. Several cases of photosensitivity caused by diltiazem have also been documented [4, 5, 6]. More recently, two reports discussed cutaneous hyperpigmentation induced by diltiazem [7, 8]. We describe the cases of a 71-year-old black male and a 49-year-old Hispanic male, who developed photodistributed hyperpigmentation during treatment with Tiazac™, a formulation of extended-release diltiazem different from any previously described.
Clinical summaries
Patient 1.—A 71-year-old black male with a past medical history of hypertension, osteoarthritis, and gout, presented with a 6-month history of progressive darkening of his face and neck. The patient had no other symptoms and did not give a history of significant sun exposure or photosensitivity. Approximately 1 year prior to presentation, the patient had begun therapy with diltiazem hydrochloride (240 mg once daily). The dosage was eventually increased to 300 mg once daily for more optimum blood pressure control. Concomitant medications included ibuprofen (400 mg twice daily) and allopurinol (300 mg once daily).
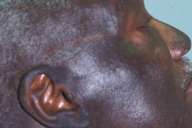
|
| Figure 1 |
|---|
| A 71-year-old black male (patient 1) with dark-brown, confluent hyperpigmentation on his face and neck. Note the sparing of areas previously covered by his glasses. |
Physical examination demonstrated dark brown hyperpigmented patches over the face and anterior and posterior neck. There was notable sparing of the areas on his face covered by his eyeglasses as well as the retroauricular areas bilaterally. A thorough physical examination revealed no other abnormalities. The results of laboratory studies, including complete blood count, liver function tests, and a comprehensive chemistry panel, were normal.
Patient 2.—A 49-year-old Hispanic male presented with a 4-month history of asymptomatic pigmented patches on his face and neck. His past medical history was significant for hypertension for which he was treated with long-acting diltiazem (240 mg once a day) for 2 years prior to presentation. He did not have any other history of inflammatory skin diseases, photosensitivity, or systemic illness before the onset of the lesions.
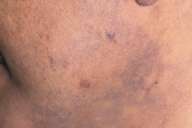
|
| Figure 2 |
|---|
| A 49 year-old Hispanic male (patient 2) with blue-gray, reticulated, hyperpigmented patches on his face and neck. |
Physical exam was significant for reticulated blue-gray hyperpigmented patches on the malar area and anterior neck, with sparing of the submental region and retroauricular areas bilaterally. A skin biopsy specimen revealed interface change with liquefaction degeneration, several necrotic keratinocytes, a few leukocytes, and melanin-laden macrophages in the superficial dermis.
Discussion
Calcium channel blockers are commonly prescribed cardiovascular agents with varied adverse cutaneous reactions. Within this heterogeneous group of drugs, nifedipine and diltiazem have been associated with skin reactions in sun-exposed areas, including photosensitive lichenoid eruptions [4], generalized erythema [5], erythematous papular skin rashes [6, 9], photodistributed facial telangiectasias [10], and photodermatoses [11].
Photodistributed hyperpigmentation is a recently described cutaneous reaction associated with diltiazem. Sherschun et al. first report four cases of diltiazem-induced hyperpigmentation of the skin. All four patients are black females who have been taking long-acting diltiazem (Cardizem CD™) for at least 6 months before developing photodistributed hyperpigmentation of the skin. In all four patients, the morphological appearance of the hyperpigmentation is reticulated and typically slate gray to blue-gray in color. Biopsies show lichenoid dermatitis with basal vacuolar alteration and prominent pigment incontinence. Dermal pigment-laden cells stain positive for melanin with Fontana-Masson and negative for iron. Followup of these patients at 1 year demonstrates the hyperpigmentation to be a reversible process after discontinuation of diltiazem and treatment with sun avoidance and hydroquinone cream. Chawla et al report similar clinical and histologic findings in a black female patient who developed slate gray hyperpigmentation of the skin confined to sun-exposed areas 3 years after starting extended-release diltiazem (Cardizem CD™).
This report extends the spectrum of patients with diltiazem-induced hyperpigmentation to include men and non-African-Americans. Our two patients and the previously reported cases share important characteristics. Each patient shows progressive hyperpigmentation in a photodistributed pattern on the face and neck. None of the patients report photosensitivity. All of the patients have been taking diltiazem for at least 6 months before the onset of hyperpigmentation. One of our patients has a prominent brown-black component to the hyperpigmentation, while the other developed a reticulated blue-gray appearance similar to that described by Sherschun et al. and Chawla et al.
Drug-induced skin pigmentation is a well-recognized effect of many different types of medications, including antimalarials, amiodarone, cytotoxic drugs, tetracyclines, heavy metals, and psychotropic drugs [12]. Clinical features are variable with a large range of patterns, color pigmentation, and distribution.
Photodistributed hyperpigmentation, in particular, has been associated with such drugs as amiodarone, minocycline, phenothiazines, and imipramine, which produce similar patterns of progressive blue-gray pigmentation in sun-exposed areas that sometimes can be differentiated histologically and histochemically. The pathogenesis of drug-induced hyperpigmentation is dependent on the causative medication and may involve an accumulation of melanin, accumulation of the drug or its metabolites, deposition of a drug-melanin complex, or deposits of iron following damage to the dermal vessels [13, 14]. Although the mechanism of diltiazem-induced hyperpigmentation is unclear, ultrastructural analysis by Scherschun et al. of their patients demonstrated fully-melanized melanosomes within dermal cells without deposits of drugs or metabolites [7]. Some authors have theorized that sun exposure may affect this process by the induction of free-radical formation of the drug or its metabolites with subsequent complex formation with melanin [15, 16]. Scherschun et al. note that, because the in vitro absorption spectrum of the diltiazem-parent compound does not have significant absorption in the UVB, UVA, and visible light ranges, another unidentified metabolite may be responsible for the photosensitivity [7]. The two primary metabolites of diltiazem are desacetyldiltiazem and desmethyldiltiazem, although as many as nine diltiazem metabolites have been identified, and total radioactivity measurements following a single intravenous dose of diltiazem suggests the presence of other unidentified metabolites [17].
Additionally, photodistributed hyperpigmentation caused by diltiazem has been associated only with the extended-release formulations. Numerous pharmacological studies have demonstrated bioequivalence of the extended-release and conventional formulations, with comparable pharmacokinetics [18, 19, 20, 21]. Extended-release formulations of diltiazem have an increase in time to maximum concentration, less fluctuation in serum diltiazem at steady state, and a prolonged half-life. In addition, Tiazac™ and Cardizem CD™ both demonstrate a more than proportional increase in diltiazem plasma concentrations as the daily dose increases [17]. However, both the extended release and conventional diltiazem formulations demonstrate nonlinear kinetics, which may predispose to accumulation of both diltiazem and its major metabolites [17, 22, 23, 24]. Therefore, from a pharmacokinetics standpoint, it remains unclear why photodistributed hyperpigmentation has been associated only with the extended release formulations and not conventional diltiazem. Given the known pharmacokinetics of diltiazem, no correlation can be made between the dosage of diltiazem and the time to onset of pigmentation on the basis of the cases reported. In two patients reported by Scherschun et al., one 49-year-old patient was exposed to a 120 mg per day dose and developed hyperpigmentation within 6 months, whereas a 72-year-old patient taking 300 mg per day developed hyperpigmentation after 11 months.
Drug-induced alterations in pigmentation are relatively common, resulting from a variety of medications, and can be a significant cosmetic concern to the patient. Hyperpigmentation caused by diltiazem is a newly reported adverse reaction. Previously, this reaction was reported only in black women, although it is now evident that it can occur in both sexes and in other ethnic groups. The appearance of the photodistributed pigmentation also varies, from reticulated slate gray or blue-gray patches to darker brown patches. There appears to be little correlation between dosage, age, and time to onset of the hyperpigmentation. Early recognition by the medical community of diltiazem-induced hyperpigmentation is important because discontinuation of the drug is a key aspect of management. The use of diltiazem in the treatment of cardiovascular diseases is common and thus it should be identified as a potential causative medication.
References
1. Wittal R, Fischer G, Georgouras K, Baird P. Skin reactions to diltiazem. Australs J Dermatol. 1992;33:11-18. PubMed2. Stern R, Khalsa J. Cutaneous adverse reactions associated with calcium channel blockers. Arch Intern Med. 1989;149:829-832. PubMed
3. Knowles S, Gupta AK, Shear NH. The spectrum of cutaneous reactions associated with diltiazem. J Am Acad Dermatol. 1998;38:201-206. PubMed
4. Hashimoto M, Tanaka S, Horio T. Photosensitivity due to diltiazem hydrochloride. Acta Dermatol. 1979;74:181-186.
5. Young L, Shehade SA, Chalmers RJ. Cutaneous reactions to diltiazem. Clin Exp Dermatol. 1990 Nov;15(6):467. PubMed
6. Seggev JS, Lagstein Z. Photosensitivity skin reactions to calcium channel blockers. J Allergy Clin Immunol. 1996 Mar;97(3):852-5. PubMed
7. Scherschun L, Lee MW, Lim HW. Diltiazem-associated photodistributed hyperpigmentation: a review of 4 cases. Arch Dermatol. 2001 Feb;137(2):179-82. PubMed
8. Chawla A, Goyal S. Diltiazem-induced hyperpigmentation in an African American woman [letter]. J Am Acad Dermatol. 2002;46:468-469.
9. Zenarola P, Gatti S, Lomuto M. Photodermatitis due to nifedipine: report of 2 cases. Dermatologica. 1991;182:196-8.
10. Collins P, Ferguson J. Photodistributed nifedipine-induced facial telangiectasia. Br J Dermatol. 1993;129:630-3. PubMed
11. Thomas SE, Wood ML. Photosensitivity associated with nifedipine. Br Med J. 1986;292:992.
12. Granstein RD, Sober AJ. Drug- and heavy metal-induced hyperpigmentation. J Am Acad Dermatol. 1981;5:1-15. PubMed
13. Crowson AN. Recent advances in the pathology of cutaneous drug eruptions. Dermatol Clin. 1999;17:537-60.
14. Lerner EA, Sober AJ. Chemical and pharmacologic agents that cause hyperpigmentation or hypopigmentation of skin. Dermatol Clin 1988;6:327-37.
15. Sicari MC. Photoinduced dermal pigmentation in patients taking tricyclic antidepressants: histology, electron microscopy, and energy dispersive spectroscopy. J Am Acad Dermatol. 1999; 40:290-3.
16. Dereure O. Drug-induced skin pigmentation. Epidemiology, diagnosis and treatment. Am J Clin Dermatol. 2001;2:253-62.
17. Thomson Healthcare, Inc. Physicians' Desk Reference, 57th ed. Montvale, NJ: Thomson Healthcare, 2003.
18. Gordin A, Pohto P, Sundberg S, Nykanen S, Haataja H, Mannisto P. Pharmacokinetics of slow-release diltiazem and its effect on atrioventricular conduction in healthy volunteers. Eur J Clin Pharmacol. 1986;31: 423-426.
19. Brorson L, Arvill A, Lofdahl P, Jorgensen E, Fraser T, Larsson H, Olsson AM, Olsson SO. Conventional and controlled release diltiazem. Bioavailability in healthy volunteers and anti-anginal effects in combination with metoprolol in stable angina pectoris. Eur J Clin Pharmacol. 1994;47:75-79.
20. Thiercelin JF, Necciari J, Caplain H, Cournot A, Combes M, Desmolin H, Flouvat B. Development and pharmacokinetics of a new sustained release formulation of diltiazem. J Cardiovasc Pharmacol. 1990;16 Suppl 1:S31-37.
21. Leeuwenkamp OR, Visscher HW, Menink CK, Jonkman JH. A comparative study of the steady-state pharmacokinetics of immediate release and controlled-release diltiazem tablets. Eur J Clin Pharmacol. 1994;46:243-247.
22. Sith MS, Verghese CP, Shand, DG, Pritchett EL. Pharmacokinetic and pharmacodynamic effects of diltiazem. Am J Cardiol. 1983;51:1396-74.
23. Caille G, Boucher S, Spenard J, Lakhani Z, Russell A, Thiffault J, Grace MG. Diltiazem pharmacokinetics in elderly volunteers after single and multiple doses. Eur J Drug Metab Pharmacokinet. 1991;16:75-80.
24. Schall R, Muller FR, Muller FO, Luus HG. Bioequivalence of controlled-release calcium antagonists. Clin Pharmacokinet. 1997:32:75-89.
© 2003 Dermatology Online Journal

