Exogenous ochronosis
Published Web Location
https://doi.org/10.5070/D33b67z1vbMain Content
Exogenous ochronosis
Joseph F Merola MD, Shane Meehan MD, Ruth F Walters MD, Lance Brown MD
Dermatology Online Journal 14 (10): 6
Department of Dermatology, New York UniversityAbstract
A 55-year-old woman with melasma develops biopsy-proved exogenous ochronosis in the setting of prolonged topical hydroquinone use. A limited number of similar reports exist in the US literature and are the basis for an FDA call to review hydroquinone-based products. This case underscores the difficult therapeutic dilemma which this diagnosis presents to dermatologists.
History
A 55-year-old-woman presented nine years ago to a dermatologist with complaints of skin darkening of her face. At that time, a diagnosis of melasma was made, and she was treated with a formulation containing hydroquinone (HQ) as its active ingredient, which the patient used twice daily for around nine months. She used several other HQ preparations for prolonged periods thereafter. The patient noticed subsequent darkening of her facial hyperpigmentation in areas and discontinued use of the topical agent. Several years later, shebegin treatment with tretinoin topical cream 0.05 percent as well as topical azelaic acid with minimal improvement in pigmentary alteration.
The patient denied history of arthritis, renal calculi/renal disease, scleral color change, or a family history of similar pigmentary alteration. A review of systems was negative.
Past medical history includes hypothyroidism for which the patient recently diagnosed diabetes for which she takes metformin.
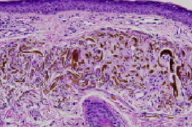 |
| Figure 1 |
|---|
Physical Examination
Hyperpigmented and gray-blue patches were distributed over the malar areas and central face.
Lab
Urinalysis was normal and no darkening of urine occurred when left to stand.
Histopathology
In the superficial dermis, there are sharply defined yellow-brown "banana" shaped deposits within homogenized elastotic fibers. There are scattered pigment-laden macrophages in the papillary dermis.
Comment
Exogenous ochronosis-like pigmentation, may occur after the topical application of hydroquinone, is limited to sites of application. The hyperpigmentation may fade slightly after discontinuing the agent, but the discoloration is usually permanent. Histopathologic examination shows yellow-brown, banana-shaped fibers in the papillary dermis. Sarcoidal granulomas with ochronotic particles in multinucleated giant cells have also been noted. In contrast to exogenous ochronosis, endogenous ochronosis or alkaptonuria, is an autosomal recessive disease, which is caused by deficiency of homogentisic acid oxidase and results in tissue accumulation of homogentisic acid, which is an insoluble pigment that binds collagen and may induce a blue-black hyperpigmentation called ochronosis. Alkaptonuria is distinguished clinically in as much as patients exhibit a multitude of systemic symptoms that are absent in the exogenous form. There features include arthritis, scleral pigmentation, cartilaginous pigmentation, black ear wax, renal calculi/failure, urine that turns dark on standing (oxidized product) and calcification and stenosis of heart valves [1].
Exogenous ochronosis most commonly results from use of hydroquinone, though resorcinol, phenol, mercury, picric acid, and antimalarials have been implicated. Hyperpigmentation typically appears after six months continual product use. The highest reported incidence of this syndrome occurs in South African Blacks. The mechanism of hyerpigmentation is speculated to involve effects on tyrosinase or alternatively by inhibiting homogentistic acid oxidase locally resulting in deposition [2].
A number of treatments have been tried with variable efficacy. Retinoic acid and sunscreen is helpful in some, dermabrasion may be beneficial; tetracycline may be helpful in sarcoid-like ochronosis. Laser therapy is reportedly effective in limited case reports. One groups reports the use of a quality-switched (QS) 755-nm alexandrite laser to treat hydroquinone-induced exogenous ochronosis in two patients. The first patient received six treatments (average fluence=7.8 J/cm²) at 2-month intervals. The second patient received four treatments (average fluence=6.9 J/cm²) at 4-month intervals. Biopsies of lesional skin were obtained before and after laser treatment for histopathologic evaluation with appreciative lightening of the pigmented skin areas achieved in both patients without scars or textural changes. Decreased dermal pigmentation was observed on histologic examination of treated skin specimens [3].
The US Food and Drug Administration proposed a ban on over-the-counter hydroquinone mainly on the basis of high absorption; reports of exogenous ochronosis in humans; and murine hepatic adenomas, renal adenomas, and leukemia with large doses over extended time periods. Systemic exposure to hydroquinone from routine topical application is no greater than that from quantities present in common food. According to at least one author, these risks are largely overstated and have no basis in human studies. Studies reveal an incidence of exogenous ochronosis in the United States of only around 22 cases in more than 50 years [4].
References
1. Snider RL, Thiers BH. Exogenous ochronosis. J Am Acad Dermatol 1993; 28: 662 PubMed2. Levin CY, Maibach H. Exogenous ochronosis: an update on clinical features, causative agents and treatment options. Am J Clin Dermatol 2001;2: 213 PubMed
3. Bellew SG, Alster TS. Treatment of exogenous ochronosis with a Q-switched alexandrite (755 nm) laser. Derm Surg, 2004; 30 : 555 PubMed
4. Levitt J. The safety of hydroquinone: a dermatologist's response to the 2006 Federal Register. J Am Acad Dermatol. 2007 Apr 26; [Epub ahead of print / article in press] PubMed
© 2008 Dermatology Online Journal

