Repigmentation in vitiligo universalis: Role of melanocyte density, disease duration, and melanocytic reservoir
Published Web Location
https://doi.org/10.5070/D338t939b8Main Content
Repigmentation in vitiligo universalis: Role of melanocyte density, disease duration, and melanocytic reservoir
Sunil Dogra MD, and Bhushan Kumar MD
Dermatology Online Journal 11 (3): 30
Department of Dermatology, Venereology and Leprology, Postgraduate Institute of Medical Education and Research, Chandigarh,
India. kumarbhushan@hotmail.com
Abstract
Recent reports suggest that vitiligo lesions are not totally devoid of melanocytes. We report an interesting observation made in the vitiliginous skin of an Indian patient being treated for pemphigus vulgaris with dexamethasone cyclophosphamide pulse therapy. Our observations indicate that melanocytes are never completely absent in the depigmented epidermis and that these melanocytes can recover their functionality in vivo and in vitro under an appropriate stimulus.
Vitiligo is an acquired disorder of pigmentation of great cosmetic concern having unpredictable course with an estimated worldwide incidence of 0.5-4 percent [1]. We report an interesting observation made in the vitiliginous skin of an Indian patient being treated for pemphigus vulgaris with dexamethasone cyclophosphamide pulse (DCP) therapy.
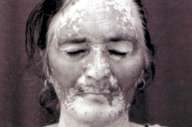
|
| Figure 1 |
|---|
| Pigmentation of the vitiliginous skin over the face. |
Clinical synopsis
A 50-year-old woman, known to have vitiligo universalis of 37-years duration, presented with history of painful oral ulceration of 4-months and vesiculobullous lesions over her body of 2-months duration. On examination, lesions were present over her face, scalp, trunk, and limbs in the form of flaccid bullae, the majority of which had ruptured leaving raw erosions. Nikolsky sign was positive and Tzanck smear from the blister showed the presence of acantholytic cells. Histopathology of a vesicular lesion was consistent with the diagnosis of pemphigus vulgaris. With this diagnosis, she was started on dexamethasone cyclophosphamide pulse (DCP) therapy. DCP regimen comprises monthly intravenous dexamethasone (140 mg) given over 3 consecutive days and intravenous cyclophosphamide (500 mg) given on day 2, and oral cyclophosphamide (1 mg/kg/day) in the intervening period. DCP regimen is a well-regarded therapy for pemphigus in India [2]. For vitiligo, she was not receiving any therapy for the last 10 years. In the past, she had received PUVA sol, systemic steroids and various topical therapies with hardly any response. Approximately 2 months after starting DCP, the patient began to develop repigmentation in the form of numerous perifollicular macules on the forearms (predominantly extensors) and shins, and diffuse pigmentation on the face. Over the next 4 months while she continued treatment with DCP for pemphigus, she continued to develop more repigmentation over her face (Fig. 1) and forearms, covering almost 25 percent of these areas . Apart from these sites, other parts of her body (especially trunk) were conspicuous by the lack of evidence of any repigmentation.
Discussion
Although the etiology of vitiligo is as yet unknown, several hypotheses have been proposed for the loss of functioning melanocytes in the skin lesions, including presence of autoantibodies against various tissues, cytotoxic T cells, autodestruction of the melanocytes by intermediates of melanogenesis pathway, oxidative stress, and the neural hypothesis [1, 2, 3]. Most of the earlier published studies in the literature reported the complete absence of melanocytes in the fully depigmented vitiliginous skin, suggesting that only hair follicles can act as a reservoir [4, 5, 6]. However, there are now reports indicating that vitiligo lesions are not totally devoid of melanocytes [7]. These observations are supported by clinical evidence such as ours, where the depigmentation process can be reversed, either spontaneously or by therapeutic approaches.
Recent a studies by Tobin et al. [8] and Bartosik et al. [9] provide a more definitive evidence that the melanocytes are still present in the depigmented epidermis of stable vitiligo of as long as 25 years. Tobin et al. [8] successfully cultured melanocytes from depigmented epidermal suction blister tissue of all the 12 randomly selected patients and these melanocytes did produce melanin. In addition, the presence of clustered and single premelanosomes in basal and suprabasal keratinocytes of lesional and normal epidermis, as well as the retention of single melanocytes in lesional epidermis, was demonstrated by light- and electron microscopy. After topical application of a narrow band UVB-activated pseudocatalase, vacuolation, granulation, and dilatation of the endoplasmic reticulum completely recovered, but the ectopic pre-melanosome shedding remained. Bartosik et al. [9] proposed that melanosomes could also persist in certain keratinocytes for some time after the onset of vitiligo. Taken together, these observations indicate that melanocytes are never completely absent in the depigmented epidermis and that these melanocytes can recover their functionality in vivo and in vitro under an appropriate stimulus. Systemic immunosuppressive drugs, including corticosteroids as given to our patient for managing pemphigus, are also recommended for the treatment of vitiligo [1]. Possibly, this could have been responsible for initiating melanocyte activity in the depigmented skin of our patient. Despite the existence of knowledge about follicular reservoir of melanocytes and the theoretical possibility of such an event as in our patients, we are not aware of any published report mentioning the reappearance of melanocyte activity in a patient of vitiligo universalis of such a long duration. Our observation supports the important findings of the above-mentioned studies.
The other important issue is the localization of repigmentation to only exposed sites in our patient i.e., face, forearms, legs, and its absence on the trunk. This can possibly be explained based on the distribution of melanocytes in various parts of the body. It is estimated that the total epidermal melanocyte population in a person is about 2 × 109 cells, there being about 2000 or more epidermal melanocytes per square millimeter on the skin of the head and forearm and usually 1000 on the rest of the body. The density of melanocytes is about twofold higher in exposed than in the non-exposed skin [3]. It has also been demonstrated that UV radiation induces pigment formation in cultured human melanocytes in vitro [11]. The total number of hair follicles in an adult person is estimated to be about 5 million, of which about 1 million are on the head; lower densities of 50-100/cm² are found on the chest and back in both sexes. A greater absolute number of melanin granules are present in the dark hair compared to the one with lighter shades. In females, generally the hair over limbs are more coarse and darker than over the trunk, so a greater reservoir for melanocytes exists in the limbs as compared to the trunk [3, 10]. Taken altogether, the above-mentioned factors, viz. increased density of melanocytes in the exposed sites, coarse black terminal hair with more melanocyte reservoir, could explain the repigmentation over these exposed sites and absence of pigmentation on the trunk.
It is possible that, in addition to the above-mentioned factors, sites constantly exposed to UV radiation as in our patient were more favorable for a repigmentation process because of stimulatory effect of the UV radiation on melanocytes. Though difficult to predict, it is likely that, with continued therapy, some degree of pigmentation will also appear on the trunk. However, It is difficult to comment upon the exact role of the baseline density, distribution of melanocytes and their functional ability in a particular patch of repigmenting vitiligo.
Numerous reports exist to document an association of vitiligo with the other autoimmune disorders. To the best of our knowledge, there are no other published reports of vitiligo coexisting with pemphigus. More importantly, it supports the observations made by earlier workers that melanocytes are never completely absent from white/ lesional vitiligo epidermis and that these cells can retain the ability to become functionally active, even after long period. This emphasizes the need for more studies in this aspect for further understanding the pathogenesis and developing better means of inducing repigmentation.
References
1. Kovacs SO, Missouri SF. Vitiligo. J Am Acad Dermatol 1998; 38: 647-666.2. Pasricha JS, Khaitan BK, Ramam RS, Chandra M. Dexamethasone-cyclophosphamide pulse therapy for pemphigus. Int J Dermatol 1995; 34:875-882.
3. Bleehen SS. Disorders of skin colour. Textbook of Dermatology. Burns DA, Breathnach SM, Champion RH, Burton JL editors, 6th edn. Oxford, Blackwell Science, 1998: pp 1753-1815.
4. Le Poole IC, van den Wijngaard RM, Westerhof W, Dutrieux RP, Das PK. Presence or absence of melanocytes in vitiligo lesions: an immunohistochemical investigation. : J Invest Dermatol 1993;100:816-822.
5. Le Poole IC, van den Wijngaard RM, Westerhof W, Das PK. Presence of T cells and macrophages in inflammatory vitiligo skin parallels melanocyte disappearance. Am J Pathol. 1996; 148: 1219-1228.
6. Arrunategui A, Arroyo C, Garcia L, Covelli C et al. Melanocyte reservoir in vitiligo. Int J Dermatol 1994; 33: 484-487.
7. Husain I, Vijayan E, Ramaiah A, Pasricha JS, Madan NC. Demonstration of tyrosinase in the vitiligo skin of human beings by a sensitive fluorometric method as well as by 14C(U)-L-tyrosine incorporation into melanin. J Invest Dermatol. 1982; 78:243-252.
8. Tobin DJ, Swanson NN, Pittelkow MR, Peters EM, Schallreuter KU. Melanocytes are not absent in lesional skin of long duration vitiligo. J Pathol 2000; 191:407-416.
9. Bartosik J, Wulf HC, Kobayasi T. Melanin and melanosome complexes in long standing stable vitiligo--an ultrastructural study. Eur J Dermatol 1998; 8: 95-97.
10. Szabo G. Regional anatomy of the human integument with special reference to the distribution of hair follicles, sweat glands, and melanocytes. Philos Trans Roy Soc Lond Biol 1967; 252: 447-485.
11. Friedmann PS, Gilchrest BA. Ultraviolet radiation directly induces pigment production by cultured human melanocytes. J Cell Physiol 1987; 133: 84-94.
© 2005 Dermatology Online Journal

