Cutaneous metastatic plasmacytomas with tropism for a previously injured limb
Published Web Location
https://doi.org/10.5070/D337n527v0Main Content
Cutaneous metastatic plasmacytomas with tropism for a previously injured limb
Marta Almeida Pereira1, Teresa Baudrier1, Alice Costa2, João Magalhães3, Filomena Azevedo1
Dermatology Online Journal 14 (9): 16
1. Departments of Dermatology and Venereology, Hospital S. João, Porto, Portugal. martapereiraderma@gmail.com2. Department of Hematology, Hospital S. João, Porto, Portugal
3. Department of Pathology, Hospital S. João, Porto, Portugal
Abstract
Cutaneous plasmacytoma is an uncommon observation in clinical practice. It is usually a consequence of direct extension from an underlying bony lesion, in the setting of multiple myeloma. In our case, a 77-year-old woman, with stage IIIA IgG λ multiple myeloma for two years, presented with firm nodular violaceous cutaneous lesions on the left arm without underlying bone osteolytic lesions or subcutaneous tumors; the biopsy was consistent with plasmacytoma. The patient had suffered two spontaneous left humeral fractures treated with prosthesis replacement just before the initial diagnosis of multiple myeloma. She had also been submitted to radiotherapy for a subcutaneous plasmacytoma, detected some months before, at the same site of the cutaneous lesions. Despite optimal response of the cutaneous lesions to treatment, the disease progressed and the patient died from infectious complications eight months after the appearance of the tumors.
Introduction
Multiple myeloma (MM) is a malignant hematologic disorder resulting from monoclonal proliferation of plasma cells. The classic diagnostic triad is marrow plasmacytosis (>10%), lytic bone lesions, and serum and urine monoclonal immunoglobulin [1-4]. Malignant plasma cells usually grow within the bone marrow, although they can proliferate in extramedullary sites, particularly the respiratory tract, oropharynx, upper gastrointestinal tract, spleen, lymph nodes, and rarely skin [3, 4]. Infiltration of the skin by malignant cells in MM gives rise to plasmacytoma, a well-recognized occurrence in this setting [1-8]. It results from direct extension to the skin from underlying osteolytic bone lesion or solitary plasmacytoma of bone. Very rarely, metastatic spreading of malignant cells without adjacent osseous lesions can occur. This usually occurs in late stages of MM when there is markedly increased tumor cell burden [1-6].
Case report
 |  |
| Figure 1 | Figure 2 |
|---|---|
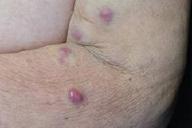
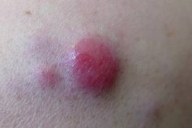
| Figure 1. Nodulo-tumoral lesions of the left upper limb Figure 2. Fleshy and violaceous tumor in detail | |
 |  |
| Figure 3 | Figure 4 |
|---|---|
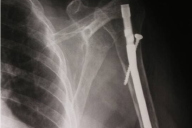
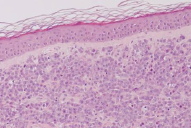
| Figure 3. Absence of osteolytic lesions Figure 4. On H&E, infiltration with dysplastic multinucleated plasma cells | |
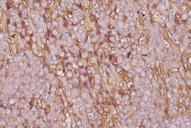
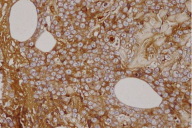
A 77-year-old woman, with a two year diagnosis of stage IIIA IgG λ multiple myeloma, presented with cutaneous nodulo-tumoral lesions of the left upper limb (Figs. 1 & 2). The tumors were non-tender, fleshy, and violaceous in color. They varied in size from 15 to 20mm (Fig. 3). No osteolytic bone involvement of the left upper limb was observed on X-ray (Fig. 4). Excisional biopsy of one lesion revealed extensive infiltration with dysplastic, abnormal multinucleated plasma cells, often with large nucleoli (Figs. 5 & 6). Immunohistochemistry showed monoclonal plasma cells expressing light lambda and gamma chains. A diagnosis of cutaneous plasmacytomas was confirmed. At this time, the bone marrow showed less than 5 percent plasma cells, but immunoelectrophoresis showed raised levels of IgG (3170 mg/dL, normal < 1560) and lambda chain (694 mg/dL, normal < 240), correlating with the immunohistochemistry profile.
 |  |
| Figure 5 | Figure 6 |
|---|---|
| Figure 5. Plasma cells expressing light lambda chains on immunohistochemistry Figure 6. Plasma cells expressing gamma chains on immunohistochemistry | |
The patient had been diagnosed with MM almost two years before. At that time, she had two consecutive pathologic fractures of the left arm and was submitted to corrective surgery and prosthesis replacement. Complaints of progressive malaise, loss of appetite, weight loss (more than 10Kg in 6 months) and generalized bone pain subsequently developed. Initial investigations showed anemia (hemoglobin concentration of 8.6 g/dL), leukopenia (2.77 x 109, normal 4 - 11 x 109/L), raised levels of urea (1.03g/L, normal 0.1-0.5), creatinine (13.6 mg/dL, normal 6 - 10), β2-microglobulin (11.1 μg/mL, normal < 2.5), lactic dehydrogenase (327 IU/L, normal 100-190), ESR (111mm/h) and C-reactive protein (1.73mg/dL, normal<0.5). Total calcium levels were normal and albumin was low (31.1g/L, normal 38-51). Immunoelectrophoresis identified a monoclonal IgG (8990 mg/dL) and lambda (1900 mg/dL) paraprotein. Free lambda chains were also present in the urine (399 mg/24h). Bone marrow plasmocytosis was approximately 44 percent. Detection of cytogenetic abnormalities with FISH (fluorescence in situ hybridization) showed 13q and 17p delections (7% of positive nuclei each). Skeletal survey showed lytic lesions in the skull, left shoulder, and ribs.
Despite a favorable clinical response to standard chemotherapy (seven cycles of melphalan and prednisolone), with normalization of the levels of hemoglobin concentration, white cell count, β2-microglobulin, lactic dehydrogenase, ESR and albumin, along with disappearance of the M component, a subcutaneous tumor subsequently developed over the left arm 18 months after diagnosis. Fine needle biopsy showed cells with excentric hypercromatic nuclei, suggestive of plasmacytoma. No medullary plasmacytosis or serum immunoelectrophoretic changes were observed and further treatment with oral cyclophosphamide (400mg/m²/week) together with local radiotherapy yielded complete regression of the tumoral mass in five weeks.
Within a few weeks, cutaneous lesions developed in areas previously submitted to local trauma, namely bone fracture and prosthesis replacement. Further treatment with cyclophosphamide (400mg/m²/week) and dexamethasone (40mg/d 4 days per month) was started and yielded a complete resolution of the cutaneous lesions in eight weeks.
Despite optimal clinical cutaneous response to treatment, the disease progressed and the patient died from infectious complications eight months after the appearance of the cutaneous plasmacytomas.
Discussion
Four types of plasma cell neoplasia are currently accepted: classic MM, extramedullary plasmacytoma without MM, solitary plasmacytoma of the bone, and plasma cell leukemia. Cutaneous involvement is documented with all types of plasma cell disorders. The most common specific cutaneous involvement by plasma cell neoplasia - plasmacytoma - is the result of direct extension to the skin from underlying bone osteolytic lesions or from metastatic spreading of malignant cells without adjacent osseous lesions [1-8].
Although soft and lymphoid tissue involvement is frequently seen in patients with classic MM and extramedullary plasmacytomas, metastatic skin lesions without adjacent bone involvement are distinctly uncommon.
Any area of the skin can be involved, but it has been reported most frequently on the trunk and abdomen, followed by the scalp, face and neck, lower extremities and upper extremities [1, 2, 4-8]. It is currently apparent that the risk of cutaneous involvement is independent of the immunoglobulin class type [3].
Our patient first presented with two humeral fractures, symptoms of general malaise, and diffuse bone pain, with no extramedullary involvement at that time. Her initial response to standard chemotherapy was quite good, with resolution of bone marrow plasmacytosis and disappearance of the blood M spike and urinary chain excretion. However, after being in remission, the patient developed a subcutaneous plasmacytoma, in the absence of either medullary relapse or serum paraprotein peak. Despite the excellent response of the subcutaneous mass to systemic treatment and local radiotherapy, cutaneous disseminated lesions subsequently developed over the left limb. This event occurred concomitantly with a paraprotein spike, reflecting progression of the disease and increased tumor cell burden. Lesional excisional biopsy of a skin lesion confirmed the presence of cutaneous spread of the disease. Histology showed that the majority of cells were plasmablasts, suggesting progression to a more malignant clone of cells.
A curious finding in this case is that the skin lesions were confined to sites where the patient had previous sustained local trauma, bone fracture and orthopedic surgery. This specific tropism to sites of previous trauma has already been reported [9, 10, 11]. This suggests trauma-specific and subcutaneous-specific plasma cell migration, possibly in relation to preferential expression of specific chemokine receptors on chemotherapy-resistant plasma cells.
Cutaneous metastasis in multiple myeloma usually indicates aggressive behavior and short survival. Also, cytogenetic features, like the presence of deletions of 13q in neoplastic plasma cells observed in our patient, indicates an unfavorable prognosis; this often indicates a poorer response to standard therapeutics [12, 13, 14].
Cutaneous plasmacytomas should be considered a sign of poor prognosis in plasma cell disorders because they generally occur late in the course of the disease. In our patient, the appearance of the skin lesions heralded a rapidly deteriorating clinical course.
References
1. Patterson JW, Parsons JM, White RM, Fitzpatrick JE, Kohout-Dutz, Rich mond and Roanoke, VA, Aurora CO. Cutaneous involvement of multiple myeloma and extramedullary plasmacytoma. J Am Acad Dermatol 1988;19:879-90 PubMed2. Daoud MS, Lust JA, Kyle RA, Pittelkow MR. Monoclonal gammapathies and associated skin disorders. J Am Acad Dermatol 1999;40:507-35 PubMed
3. Jorizzo JL, Gammon WR, Briggaman RA. Cutaneous plasmacytomas. A review and presentation of an unusual case. J Am Acad Dermatol 1979;1:59-66 PubMed
4. Requena L, Kutzner H, Palmedo G, Calonje E, Requena C, Pérez G, Pastor MA, Sangueza OP. Cutaneous involvement in multiple mieloma. Arch Dermatol 2003;139:475-86 PubMed
5. Tüting T, Bork K. Primary plasmacytoma of the skin. J Am Acad Dermatol 1996;34:386-90 PubMed
6. Shpilberg O, Yaniv R, Levy Y, Trau H, Ben-Bassat I. Huge cutaneous plasmacytomas complicating multiple myeloma. Clin Exp Dermatol 1994; 19:324-26 PubMed
7. Patel K, Carrington PA, Bhatnagar S, Houghton JB, Routledge RC. IgD myeloma with multiple cutaneous plasmacytomas. Clin Lab Haem 1998; 20: 53-55 PubMed
8. Kato N, Kimura K, Yasukawa K, Aikawa K. Metastatic cutaneous plasmacytomas: a case report associated with IgAλ multiple myeloma and review of the literature of metastatic cutaneous plasmacytomas associated with multiple myeloma and primary cutaneous plasmacytomas. J Dermatol 1999;26:587-94 PubMed
9. Rosenblum MD, Bredeson CN, Chang C, Rizzo JD. Subcutaneous plasmacytomas with tropism to sites of previous trauma in a multiple myeloma patient treated with autologous bone marrow transplant. Am J Hematol 2003;72:274-77 PubMed
10. Trullemans F, Schots R, Storme G, Camp BV. Late and localized extramedullary relapse of a light chain κ myeloma after syngeneic bone marrow transplantation. Bone Marrow Transplant 2000;25:115-117 PubMed
11. Kerob D, Vantelon JM, Ribrag V, Bosq J, Desruennes E, Bourhis JH, Avril MF. Localisations cutanées d'un myeloma sur le traject des voies veineuses centrales. Ann Dermatol Venereol 2002;129:311-14 PubMed
12. Shaughnessy J Jr, Tian E, Sawyer J, McCoy J, Tricot G, Jacobson J, Anaissie E, Zangari M, Fassas A, Muwalla F, Morris C, Barlogie B. Prognostic impact of cytogenetic and interphase fluorescence in situ hybridization-defined chromosome 13 delection in multiple myeloma: early results of total therapy II. Br J Hematol 2003;120:44-52 PubMed
13. Tricot G, Sawyer JR, Jagannath S, Desikan KR, Siegel D, Naucke S, Mattox S, Bracy D, Munshi N, Barlogie B. Unique role of cytogenetics in the prognosis of patients with mieloma receiving high dose therapies and autotransplants. J Clin Oncol 1997; 15:2659-66 PubMed
14. Tricot G, Barlogie B, Jagannath S, Bracy D, Mattox S, Vesole DH, Naucke S, Sawyer JR. Poor prognosis in multiple mieloma is associated only with partial or complete deletions of cromossome 13 or abnormalities involving 11q and not with other karyotype abnormalities. Blood 1995; 86:4250-6 PubMed
© 2008 Dermatology Online Journal

