|
|
| Figure 1 |
Figure 2 |
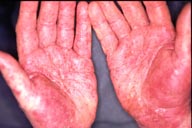
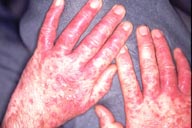
Figures 1 and 2: Pustular psoriasis at palms and dorsal hands 3 days after starting Skin Cap spray.
A 53 year-old male with a history of chronic stable plaque-type psoriasis presented with increasing erythema, induration and
pustules at hands, abdomen and thighs 3 days after starting Skin Cap spray. He also noted darkening of his urine and fatigue.
He was on no other medications and otherwise had a non-contributory past medical history. In particular, he denied a past
history of pustular psoriasis and hepatitis. Review of systems revealed an alcohol intake of 2-3 bottles of beer per week
and wine intake of 1- 2 glasses nightly. He denied a recent history of upper respiratory tract illness, flu-like symptoms
or shellfish ingestion. He had no past history of allergic contact dermatitis to any topical medications or shampoos. He had
discontinued Skin Cap on the 4th day and was assessed 5 days later. Physical examination revealed erythematous patches studded
with micropustules and hands (see figure), forearms, abdomen and upper thighs involving approximately 15 0f total body surface
area . He was afebrile and no obvious scleral icterus was noted. Laboratory investigations revealed AST 165 (reference range
<37) U/L, ALP 183 (reference range 50-130) U/L, total bilirubin 19.4 (reference range <18) U/L. Screening serology for hepatitis
A, B and C were negative as were ANA (anti-nuclear antibody) and ENA (extractable nuclear antigens). Management consisted
of cool compresses and halobetosol propionate ointment bid. At 10 day follow-up, significant improvement with marked reduction
in pustular erythema was noted along with normalization of liver function tests. The patient declined to have repeat open
application patch testing of Skin-Cap spray. Patch testing to individual Skin-Cap ingredients was not performed.
Discussion
Skin Cap is a topical preparation containing zinc pyrithione (0.2%), sodium methyl ethyl sulfate (0.01%), isopropyl myristate,
alcohol, and isobutane. Of these ingredients, sodium lauryl sulfate and alcohol are potential irritants and occasional allergens
while zinc pyrithione and isopropyl myristate are rarely contact allergens. Sodium lauryl sulfate, an emulsifyng agent, can
enhance the percutaneous penetration of other substances including other potential irritants and allergens. Alcohol has also
been implicated in systemic contact dermatitis where ingestion of alcohol by individuals sensitized by external contact may
lead to extensive eczematization (4). Furthermore, alcohol is a known hepatotoxin and potential aggravating factor in psoriasis.
The temporal association of Skin Cap use and development of pustular psoriasis along with symptoms and laboratory evidence
of hepatotoxicity suggest a probable linkage in these events. Against the background of pre-existing alcohol intake in this
patient, it is possible that the additional alcohol content of Skin Cap may have directly precipated pustular psoriasis and
hepatotoxicity in this patient. Alternatively, an ingredient in Skin Cap may have resulted in topical sensitization resulting
in a systematized Koebner response with subsequent widespread pustular psoriasis and hepatotoxicity.
This case serves to illustrate the importance of maintaining vigilance for the possibility of adverse events developing in
over-the-counter preparations which patients may presume to be of negligible adverse risk. In the case of Skin Cap, warning
the patient about the possibilty of this adverse reaction and concern about interaction with ingested alcohol may be appropriate.
Jerry K. L. Tan, MD, FRCP
References 1. Gallego H. Letter 111. Dr. Gallego Adds. Schoch Letter 1997; 47:26.
2. Crutchfield CE, Lewis EJ, Zelickson BD. The Effective Use of Topical Zinc Pyrithione in the Treatment of Psoriasis: A Report
of Three Cases. J Geriatr Dermatol 1997; 5:21-4.
3. Shelley WB, Shelley ED. Portrait of a Practice. Cutis 1997;59:181-2.
4. Fisher AA. Contact Dermatitis. Philadelphia: Lea-Febiger; 1986.
|



