Lichenoid eruption induced by etanercept
Published Web Location
https://doi.org/10.5070/D32fw0w71nMain Content
Letter: Lichenoid eruption induced by etanercept
Nuria Barrientos MD, Sagrario García-Sánchez MD, José D Domínguez MD
Dermatology Online Journal 18 (7): 15
1. Department of Dermatology, Hospital del Henares, Coslada, Spain2. Department of Pathology, Hospital del Henares, Coslada, Spain
Abstract
Lichenoid drug eruption is an uncommon, but previously reported, side effect of anti-tumor necrosis factor therapy. The majority of these adverse events relate to infliximab. We report a patient who developed a lichenoid eruption on the back of her hands during etanercept therapy. She improved with topical treatment and discontinuation of the drug was not necessary. The physiopathological link between anti-TNF treatment and lichenoid eruptions remains unclear. It is important to realize that a lichenoid reaction pattern may occur during anti-TNF agent treatment.
Case report
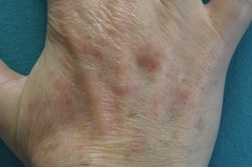
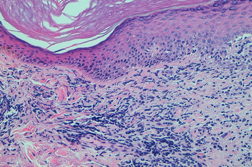
A 53-year-old woman with severe rheumatoid arthritis since 2008, with positive rheumatoid factor, was treated with oral corticosteroids, methotrexate, adalimumab, and leflunomide. Because of the ineffectiveness of previous treatments, the patient started with etanercept (50 mg/week) in November, 2010. After two months, she presented with erythematous-violaceous papular lesions symmetrically located on the dorsa of both hands and wrists (Figure 1). She complained of mild pruritus. There was no mucosal or nail involvement. A biopsy specimen was taken from the back of the right hand and a histological examination revealed confluent hyperkeratosis with irregular acanthosis, basal vacuolization with focal apoptotic cells in the epidermis, and diffuse inflammatory lymphocytic infiltrate in the superficial dermis (Figure 2).
The cutaneous lesions were treated with topical therapy (mometasone fuorate cream 0.1% once per day). As of this writing, etanercept has not been discontinued and the eruption has improved with the topical treatment.
Discussion
The cutaneous adverse events induced by etanercept have been described as injection-site reactions, eczematous skin lesions, vascular lesions, erythema multiforme, urticaria-like reactions, skin infections, lupus erythematosus, alopecia areata, non-melanoma skin cancer, and licheoid eruptions [1].
The tumor necrosis factor (TNF-α) inhibitors have been implicated in several cases of lichenoid eruptions. The majority of these adverse events relate to infliximab [2]. Five cases have been described with etanercept to date. Two patients were being treated with etanercept for rheumatoid arthritis [3, 4], two for psoriatic arthritis [1, 5], and one for psoriasis [6]. The clinical morphology described in these cases was varied, but oral involvement was not reported. Two cases showed lesions on the back of the hands quite similar to our patient [5, 6]. The time from initiation of etanercept therapy and the onset of eruption varied from 5 weeks [6] to several months [1]. The etanercept therapy was stopped in all cases. Complete recovery was reported in four cases.
The physiopathological link between anti-TNF treatment and lichenoid eruptions remains unclear. Some authors propose that inhibition of TNF-α allows for up-regulation of other precursor cytokines such as interferon-α. Interferon-α then induces activation of resident T cells and myeloid dendritc cells and a subsequent inflammatory response [2]. On the other hand, there have been reports of lichen planus treated successfully with etanercept [7]. A similar phenomenon can be hypothesized for psoriasis; despite their known anti-psoriatic efficacy, anti-TNF agents may induce psoriasiform lesions in a subset of cases [8]. Further research is needed to elucidate the pathogenesis.
When a TNF-α inhibitor causes a lichenoid eruption it is often recommended to stop the treatment but this decision will need to be made on an individual basis, based on the extent of the eruption and severity of the patient’s underlying disease.
References
1. Garcovich S, Manco S, Zampetti A et al. Onset of lichen planopilaris during treatment with etanercept. Br J Dermatol. 2008 May;158(5):1161-1163. [PubMed]2. Asarch A, Gottlieb A, Lee J et al. Lichen planus-like eruptions: An emerging side effect of tumor necrosis factor-α antagonists. J Am Acad Dermatol. 2009 Jul;61(1):104-111. [PubMed]
3. Battistella M, Rivet J, Bachelez H et al. Lichen planus associated with etanercept. Br J Dermatol. 2008 Jan;158(1):188-190. [PubMed]
4. Flendrie M, Vissers WH, Creemers MC et al. Dermatological conditions during TNF-alpha-blocking therapy in patients with rheumatoid arthritis: a prospective study. Arthritis Res Ther. 2005 Apr;7(3):R666-676. [PubMed]
5. Garcovich S, Burlando M, Rongioletti F et al. Cutaneos drug eruption with an Interface Dermatitis Pattern due Anti-tumor Necrosis-Factor-alpha Agents: A Relevant Class-effect. Acta Derm Venereol. 2010 May;90(3):311-312. [PubMed]
6. Bovenschen HJ, Kop EN, Van De Kerkhof PC et al. Etanercept-induced lichenoid reaction pattern in psoriasis. J Dermatolog Treat. 2006 Dec;17(6):381-383. [PubMed]
7. Yarom N. Etanercept for the manegement of oral lichen planus. Am J Clin Dermatol. 2007;8(2):121. [PubMed]
8. Moustou AE, Matekovits A, Dessinioti C et al. Cutaneous side effects of anti-tumor necrosis factor biologic therapy: A clinical review. J Am Acad Dermatol. 2009 Sep;61(3):486-504. [PubMed]
© 2012 Dermatology Online Journal