Pseudo-Kaposi sarcoma: A challenging vascular phenomenon
Published Web Location
https://doi.org/10.5070/D32df3x9t0Main Content
Pseudo-Kaposi sarcoma: A challenging vascular phenomenon
Sibel Doğan MD, Gonca Boztepe MD, Ayşen Karaduman MD
Dermatology Online Journal 13 (3): 22
Hacettepe University Faculty of Medicine Department of Dermatology, Ankara, TurkeyAbstract
Pseudo-Kaposi sarcoma is a rare vascular phenomenon which can be related to congenital vascular malformations or acquired venous insufficiency. The differential diagnosis includes Kaposi sarcoma. In this report, a patient with Pseudo-Kaposi sarcoma Stewart-Bluefarb subtype is presented and the related literature is reviewed.
Clinical synopsis
A 17-year-old boy who suffered from confluent vascular plaques on the left leg since birth was admitted for evaluation. He was complaining about the progression of the lesions over the last year. The lesions were associated pain and edema and they prevented him from standing for a long time.
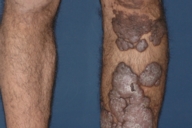
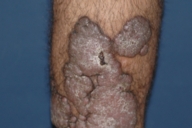
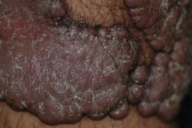
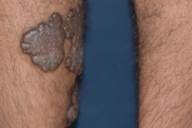
Dermatologic examination revealed hypertrophic, violaceous plaques around the left ankle spreading to the proximal part of the knee. Up to five satellite firm lesions on the anterior tibia were noted. The plaque continued as a red-purple patch on the mediolateral of the distal 1/3 of the thigh. Neither ulceration nor elevation in the skin temperature were observed, however an enlargement both in the thickness and length of the involved limb was prominent. Although bilateral femoral pulses were palpable, the left popliteal pulse was absent. The rest of the dermatologic and physical examination were non-remarkable (Figs. 1-4).
 |  |
| Figure 1 | Figure 2 |
|---|---|
| Figure 1. Patient's leg with vascular plaques is obviously thicker than the other one. Figure 2. Vascular hypertrophic plaques on patient's left leg. | |
 |  |
| Figure 3 | Figure 4 |
|---|---|
| Figure 3. Hypertrophic, violaceous plaques are seen nearby. Figure 4. Hypertrophic plaques are observed on posterior aspect of the leg. | |
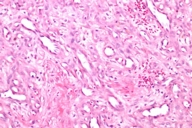
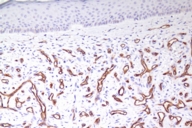
The patient was given a presumptive diagnosis of pseudo-Kaposi sarcoma. A shave biopsy was performed on one of the satellite lesions. Histopathologic examination showed dilated capillaries, extravasated erythrocytes and surrounding lobular hyperplastic granulation tissue. Vascular slits and CD34 expression were absent, which confirmed the clinical diagnosis of pseudo-Kaposi sarcoma. Figures 5 and 6 demonstrate the histologic features that enabled us to rule out the differential diagnosis like Kaposi sarcoma and other malign vascular proliferations.
On his second examination about a week after the biopsy, we surprisingly observed that the patient had developed an additional hypertrophic satellite lesion at the shave biopsy site. He was later evaluated by the department of cardiothoracic surgery and angiographic embolization of arteriovenous malformations enhanced by Doppler USG was considered as the treatment of choice.
Discussion
Pseudo-Kaposi sarcoma is a rare self limited disorder that can be congenital (as in our case) or acquired. The patients develop brown macules, violaceous or purplish nodules and plaques which become verrucous or ulcerate. Pseudo-Kaposi sarcoma has been reported in patients who had chronic venous insufficiency [1], paralyzed extremities [2], amputation stump [3], procedures of arteriovenous vascular fistula for hemodialysis [4], and suction-socket lower limb prosthesis [5]. There are also reports claiming minor traumas as a cause of pseudo-Kaposi sarcoma[6].
Although it was Earhart et al. who first used the term pseudo-Kaposi sarcoma in 1974 [7], this clinical picture was first reported by Mali in 1965 as a kaposiform skin lesion in patients with chronic vascular insufficiency. Angiodermatitis of Mali refers to the proliferation of vessels of the lower extremities, mainly on the dorsal aspect of the feet and ankles as a result of chronic venous insufficiency. The lesions particularly appear in elder patients in the background of stasis dermatitis [1]. In 1967 Bluefarb and Adams described a similar type of skin lesion in a patient who had congenital arteriovenous malformation and who did not have venous insufficiency or stasis dermatitis [8]. An arteriovenous malformation beginning early in life that unilaterally involves the lower extremities is the characteristic of the described clinical presentation also known as Stewart-Bluefarb syndrome. The underlying arteriovenous shunt can be diagnosed by the presence of a palpable thrill or audible bruit. Stewart-Bluefarb syndrome was recently suggested to be secondary to Klippel-Trenaunay syndrome. Unlike this syndrome Stewart-Bluefarb syndrome is seldom accompanied with limb hypertrophy [1].
Our patient was diagnosed as Stewart-Bluefarb syndrome on the basis of his history, dermatological and histopathological examination. Stewart-Bluefarb syndrome is characterized by unilateral involvement of a lower extremity associated with an underlying arteriovenous malformation. Patients may have pain, increased warmth, and enlargement of the limb. The lesions can progress resulting in ulcer formation, infection, and bleeding.
We know that the high oxygen saturation and high perfusion might lead to neovascularization and fibroblast proliferation and the endothelial cell proliferation may be stimulated by local factors such as elevated venous pressure, retrograde blood flow and edema [9]. These features also refer to the interesting finding in our patient which is the immediate hypertrophy at the excision site. It has also been reported that distal hypoxia may induce endothelial proliferation through a local increase of vascular endothelial growth factor (VEGF) . We believe that this effect might have also played a role in the hypertrophy of the excised portion of the lesion in our patient [10].
Histopathologically, pseudo-Kaposi sarcoma differs from Kaposi sarcoma by the lack of characteristic Kaposi sarcoma's vascular slits. The expression of CD34 antigen is a feature of Kaposi sarcoma and is not observed in pseudo-Kaposi sarcoma. Factor VIII- associated antigen is seen in Pseudo-Kaposi lesions, however they are absent in Kaposi sarcoma. A search for HHV-8 in the lesional skin which is a characteristic feature in Kaposi sarcoma can also be helpful in differential diagnosis [11]. The differential features of Kaposi and pseudo-Kaposi sarcoma are given in Table 1.
Diagnostic measure in pseudo-Kaposi sarcoma include Doppler USG and arteriography the latter being the gold standard. The angiographic hallmark of an arteriovenous malformation is early venous filling proportional to the extent of the arteriovenous connections. Radionuclide 99 m Tc labeled sodium scan is also used suggesting a less invasive method compared to angiography [9]. For our patient our therapeutic approach consisted of angiography and cardiothoracic surgery consultation for embolization of the arteriovenous malformations.
Complications such as invasion, infection, hemorrhage, limb hypertrophy, bone demineralization and congestive heart failure makes the issue harder to manage. Also the invasive treatment modalities could increase the morbidity. Our experience shows neither partial excision nor surgical correction methods are contraindicated because of the lesion's natural high capability of neovascularization and regeneration. Conservative treatment is preferred when numerous small arteriovenous shunts are present. Selective embolization can be performed. Unfortunately in complicated cases amputation is still the last choice of treatment. It is also important that specialists should consider of underlying arteriovenous malformations in young patients with pseudo-Kaposi sarcoma. In conclusion, we wanted to share our experience on a rare and challenging case of Stewart-Bluefarb syndrome and review the recent literature on pathogenesis, clinic, histopathology and the therapeutic options of the disease.
References
1. Mali JWH et al. Acro-angiodermatitis of the foot. Arch Dermatol 1965; 92: 515-518.2. Landthaler M et al. Mali's acroangiodermatitis (pseudo-Kaposi) in paralyzed legs. Hautarzt. 1988; 39 (5): 304-7.
3. Hodl S et al. Kaposiform angiodermatitis (pseudo-Kaposi disease) on an amputation stump. A new entity. Hautzart 1988; 39 (5): 302-303.
4. Goldblum OM et al. Pseudo-Kaposi sarcoma of the hand associated with an acquired, iatrogenic arterovenous fistula. Arch Dermatol. 1985 Aug;121(8):1038-40.
5. Sbano et al. Acroangiodermatitis (pseudo-Kaposi sarcoma) associated with verrucous hyperplasia induced by suction-socket lower limb prosthesis. J Cutan Pathol 2005: 32: 429-432.
6. Del-Rio E et al. Pseudo-Kaposi sarcoma induced by minor trauma in a patient with Klippel-Trenaunay-Weber syndrome. Clin Exp Dermatol. 1993 Mar;18(2):151-3.
7. Earhart RN et al. Pseudo-Kaposi sarcoma. A patient with arteriovenous malformatin and skin lesions simulating Kaposi sarcoma. Arch dermatol. 1974; 110: 907-910.
8. Bluefarb SM et al. Arteriovenous malformation with angiodermatitis. Stasis dermatitis simulating Kaposi sarcoma. Arch Dermatol 1967; 96: 176-181.
9. Smiddy PF et al. Pseudo-Kaposi sarcoma: The association of arteriovenous malformations with skin lesions resembling Kaposis's sarcoma. Australas Radiol. 2001; 45: 225-227.
10. Brenner S et al.What's new in pseudo-Kaposi sarcoma? JEADV 2001; 15: 382-384.
11. Murakami et al. Factor XIIIa expression in pseudo-Kaposi sarcoma.J Dermatol. 1991 Nov;18(11):661-6.
© 2007 Dermatology Online Journal