Segmental neurofibromatosis in association with a large congenital nevus and malignant melanoma
Published Web Location
https://doi.org/10.5070/D324d117scMain Content
Segmental neurofibromatosis in association with a large congenital nevus and malignant melanoma
Sean D Doherty 1, Saira George MD2, Victor G Prieto MD PhD3, Jeffrey E Gershenwald MD4, Madeleine Duvic MD5
Dermatology Online Journal 12 (7): 22
1. Baylor College of Medicine, Houston, TX2. Department of Dermatology, Baylor College of Medicine, Houston, TX 3. Departments
of Dermatology and Pathology, The University of Texas, MD Anderson Cancer Center, Houston, TX 4. Department of Surgical Oncology,
The University of Texas, MD Anderson Cancer Center, Houston, TX 5. Department of Dermatology, The University of Texas, MD
Anderson Cancer Center, Houston, TX. mduvic@mdanderson.org
Abstract
We describe a patient with segmental neurofibromatosis and a large congenital nevus who developed malignant melanoma in the same anatomic distribution. The overlapping presentation suggests a common etiologic link between neurofibromatosis and large congenital nevi and the possibility that neurofibromatosis increases the risk of melanoma with large congenital nevi.
Clinical synopsis
A 48-year-old man with segmental neurofibromatosis (NF) was evaluated for a melanoma arising within a large congenital nevus (LCN) on the upper trunk. During childhood the patient began developing numerous small, rubbery nodules within the nevus. He also had a congenital fusion of the cervical spine. At age 16 he underwent excision of a cervical plexiform neurofibroma. About 4 months prior to presentation, he noted an area of color change and irritation within the nevus; this was later determined to be melanoma. There was no family history of melanoma or neurofibromatosis.

|
|
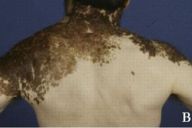
| Figure 1A | Figure 1B |
|---|---|
| The patient's extensive large congenital nevus. The large soft tissue mass in the left cervical area is attributed to an underlying plexiform neurofibroma. | |
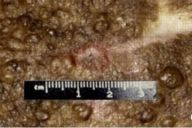
|
| Figure 1C |
|---|
| Scattered papules and nodules within the nevus |
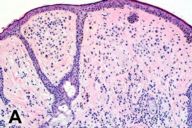
Examination of the skin demonstrated an extensive darkly pigmented nevus covering his neck, shoulders, upper back, and chest, with greater involvement of the left side than the right (Figs. 1A and 1B). The nevus itself contained several irregularly raised and hyperpigmented areas as well as numerous scattered, soft, rubbery papules and nodules ranging in size from several millimeters to 2 centimeters (Fig. 1C). No café au lait spots, axillary freckling, neurofibromas, or other irregularities were noted to involve the skin outside of the nevus. Lisch nodules were not found by slit-lamp exam. The remainder of the exam was significant for a large soft-tissue swelling underlying the nevus in the left cervical region and for marked kyphosis and scoliosis.

|
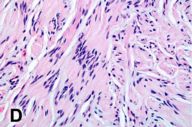
|
| Figure 2C | Figure 2D |
|---|---|
|
Figure 2C. One of the nodules within the large congenital nevus (2x) Figure 2D. High power of 2C. Notice the hyper- and hypo-cellular areas in the lesion (Antoni A) (20x). |
|
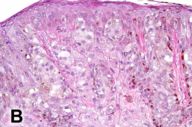
Several pathology specimens were taken from the large pigmented truncal lesion. Some revealed an intradermal melanocytic nevus with mature melanocytes located predominantly in the dermis (Fig. 2A). The deeper areas of the nevus showed scattered areas of spindle type-C nevus cells located around skin adnexa in a pattern characteristic of congenital nevi. Other areas contained melanoma, characterized by atypical, large epithelioid cells with pagetoid upward migration in the epidermis (Fig. 2B) and dermal involvement. A biopsy specimen taken from within the congenital nevus demonstrated a well-circumscribed proliferation of spindle cells with Antoni A and B areas, features characteristic of a schwannoma (figures 2c and d).
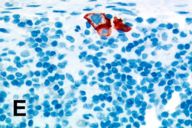
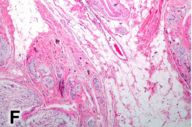
A sentinel lymph node from the left posterior cervical region sampled at the time of the melanoma excision revealed a few melanoma cells only within the subcapsular area, a finding confirmed with an immunohistochemical study with HMB45 (Fig. 2E). A separate biopsy of the large neck mass obtained during a left posterolateral neck dissection showed a plexiform proliferation of spindle cells within a myxoid stroma, i.e., a plexiform neurofibroma (Fig. 2F). A smaller subcutaneous neurofibroma was also excised at that time. The patient died 8 months after initial presentation form complications secondary to metastatic melanoma.
The diagnosis of segmental neurofibromatosis type 1 (NF1) is based on the co-existence of a plexiform neurofibroma in the left cervical region, and a subcutaneous neurofibroma in the same area. Segmental NF1 results from somatic mosaic mutations of the NF1 gene [1]. A single plexiform neurofibroma is sufficient to diagnose segmental NF1 [2] because these tumors are almost exclusively associated with neurofibromatosis [3, 4] and have been shown to arise from an early embryonic loss of heterozygosity at the NF1 locus [5]. Plexiform neurofibromas generally affect not only the nerve itself but also the development of the entire surrounding segment or region of the body. Abnormal soft tissue hypertrophy or dysplastic skeletal lesions, such as the spinal abnormalities seen in our patient, often accompany plexiform neurofibromas [6, 7]. Although hyperpigmentation and hypertrichosis of the skin overlying plexiform neurofibromas are frequently reported[6,7], this patient had also a congenital nevus. In a recent paper, Happle proposed that both the plexiform neurofibroma and the associated dysplastic changes in the regional tissue are a manifestation of a common underlying segmental mutation of the NF1 gene [8].
The NF1 gene encodes a protein, neurofibromin, that has been shown to have effects related to both melanogenesis and melanoma. Neurofibromin modulates regulation of tyrosinase[9] and tyrosinase related protein-2[10], two proteins involved in melanogenesis. The characteristic findings of Lisch nodules of the iris, café au lait spots, and axillary freckling in NF1 indicate that mutant NF alleles result in abnormal melanogenesis [10]. Interestingly, this may have also played a role in the formation of the LCN overlying this patient's plexiform neurofibroma.
A link between NF1 and LCN is supported by the significantly higher frequency of LCN in the NF1 population. LCN are estimated to occur in 1 in 20,000 newborns in the general population [11]. Reviews of patients with NF report that 1.2-3.6 percent of patients have LCN [12,13,14], representing a 240-720-fold increased risk of LCN in patients with NF.
The occurrence of melanoma with neurofibromatosis suggests that an NF1 mutation may promote its development. Neurofibromin has a GTPase-activating protein-like domain[15] and downregulates the p21ras proto-oncogene product by converting active GTP-bound p21ras to its inactive GDP-bound state [16]. N-ras oncogene mutations have been found in both congenital nevi and melanoma [17]. Additionally, NF1 mutations have been detected in melanoma cell lines [18, 19] and some melanoma subtypes[20], but data from case reports and large studies of cancers in NF patients suggests that melanoma is a rare malignant complication of NF1 [21, 22]. NF1 inactivation, therefore, is likely not an initiating factor in the malignant transformation of nevus cells, but it could contribute to later steps of melanoma progression [18].
Neurofibromatosis type 1 is also associated with tumors of other neural crest-derived cells such as Schwann cells. A list of other tumors associated with NF1 is shown in Table 1. Schwannomas rarely occur in the skin, but benign and malignant schwannomas have been reported to occur in LCN [23, 24]. A spectrum of neural crest lesions encountered within close proximity to each other in this patient, including a plexiform neurofibroma, schwannoma, nevus, and melanoma, suggests their development may be related.
The effect of NF1 mutations on melanoma risk in patients with both NF1 and LCN is not known. In the absence of NF1, LCN is a well-recognized risk factor for melanoma with an estimated incidence of 4.6- 6.3 percent [28, 29]. In addition to our patient, 3 of the 4 (75 %) NF patients with LCN reported by Brasfield[12] and 4 of the 15 (26 %) NF patients with LCN reviewed by Rubinstein[14] developed melanoma; therefore, patients with both NF and LCN could be at a higher risk of developing melanoma than those with either NF or LCN alone.
References
1. Tinschert S, Naumann I, Stegmann E, et al. Segmental neurofibromatosis is caused by somatic mutation of the neurofibromatosis type 1 (NF1) gene. Eur J Hum Genet. 2000; 8: 455-9. PubMed2. Ruggieri M, Huson SM. The clinical and diagnostic implications of mosaicism in the neurofibromatosis. Neurology. 2001; 56(11): 1433-43. PubMed
3. Huson SM, Ruggieri M. The neurofibromatoses. In Harper J, Oranje JM, Prose N, eds. Pediatric Dermatology. Malden, MA: Blackwell Science; 2000, 1204-1224.
4. McCarron KF, Goldblum JR. Plexiform neurofibroma with and without associated malignant peripheral nerve sheath tumor. Mod Pathol. 1998; 11: 612-7. PubMed
5. Kluwe L, Friedrich RE, Mautner VF. Allelic loss of the NF1 gene in NF1-associated plexiform neurofibromas. Cancer Genet Cytogenet. 1999; 113: 65-9. PubMed
6. Riccardi VM. Neurofibromatosis: phenotype, natural history, and pathogenesis. Baltimore: Johns Hopkins University Press; 1992.
7. Woodruff JM. Pathology of tumors of the peripheral nerve sheath in type 1 neurofibromatosis. Am J Med Genet. 1999; 89: 23-30. PubMed
8. Happle R. Large plexiform neurofibromas may be explained as a type 2 segmental manifestation of neurofibromatosis 1. Am J Med Genet. 2001; 98: 363-364. PubMed
9. Suzuki H, Takahashi K, Yasumoto K, Shibahara S. Activation of the tyrosinase gene promoter by neurofibromin. Biochem Biophys Res Commun. 1994; 205(3): 1984-91. PubMed
10. Suzuki H, Kazuhiro T, Yasumoto K, et al. Role of neurofibromin in modulation of expression of the tyrosinase-related protein 2 gene. J Biochem (Tokyo). 1998; 124(5): 992-8. PubMed
11. Castilla EE, da Graca Dutra M, Orioli-Parreiras IM. Epidemiology of congenital pigmented naevi: I. Incidence rates and relativefrequencies. Br J Dermatol. 1981; 104: 307-15. PubMed
12. Brasfield RD, Das Gupta TK. Von Recklinghausen's disease: a clinicopathological study. Ann Surg. 1972; 175: 86-104. PubMed
13. Crowe FW, Schull WJ, Neel JV. A clinical, pathological and genetic study of multiple neurofibromatosis. Springfield, IL: Charles C Thomas Publishers, 1956.
14. Rubenstein AE, Seitz SC, Wallace S, et al. Increased risk of congenital premalignant melanocytic nevi in neurofibromatosis. Neurology. 1985; 35 (Suppl 1): 194.
15. Xu GF, O'Connell P, Viskochil D, et al. The neurofibromatosis type 1 gene encodes a protein related to GAP. Cell. 1990; 62(3): 599-608. PubMed
16. Bollag G, McCormick F. Ras Regulation. NF is enough of GAP. Nature. 1992; 356 (6371): 663-4. PubMed
17. Carr J, Mackie RM. Point mutations in the N-ras oncogene in malignant melanoma and congenital naevi. Br J Dermatol. 1994; 131(1): 72-7. PubMed
18. Andersen LB, Fountain JW, Gutmann DH, et al. Mutations in the neurofibromatosis 1 gene in sporadic malignant melanoma cell lines. Nat Genet. 1993; 3: 118-21. PubMed
19. Johnson MR, Look AT, DeClue JE, et al. Inactivation of the NF1 gene in human melanoma and neuroblastoma cell lines without impaired regulation of GTP.Ras. Proc Natl Acad Sci U S A. 1993; 90: 5539-43. PubMed
20. Gutzmer R, Herbst RA, Mommert S, et al. Allelic loss at the neurofibromatosis type 1 (NF1) gene locus is frequent in desmoplastic neurotropic melanoma. Hum Genet. 2000; 107: 357-61. PubMed
21. Zoller ME, Rembeck B, Oden A, et al. Malignant and benign tumors in patients with neurofibromatosis type 1 in a defined Swedish population. Cancer 1997; 79: 2125-31. PubMed
22. Duve S, Rakoski J. Cutaneous melanoma in a patient with neurofibromatosis: a case report and review of the literature. Br J Dermatol. 1994; 131: 290-4. PubMed
23. Hendrickson MR, Ross JC. Neoplasms arising in congenital giant nevi: morphologic study of seven cases and a review of the literature. Am J Surg Pathol. 1981; 5(2): 109-35. PubMed
24. Jerdan MS, Cohen BA, Smith RR, Hood AF. Neuroectodermal neoplasms arising in congenital nevi. Am J Dermatopathol 1985; 7 Suppl: 41-48. PubMed
25. Yohay K. Neurofibromatosis types 1 and 2. Neurologist. 2006 Mar;12(2):86-93. PubMed
26. Fernandez MT, Puig L, Capella G, et al. Von Recklinghausen neurofibromatosis with carcinoid tumor and submucous leiomyomas of the duodenum. Neurofibromatosis. 1989;1:294-8. PubMed
27. Guillot B, Dalac S, Delaunay M, et al. Melanoma Res. 2004 Apr;14(2):159-63. PubMed
28. Lorentzen M, Pers M, Bretteville-Jensen G. The incidence of malignant transformation in giant pigmented nevi. Scand J Plast Reconstr Surg. 1977; 11(2): 163-7. PubMed
29. Rhodes AR, Wood WC, Sober AJ, Mihm MC Jr. Nonepidermal origin of malignant melanoma associated with a giant congenital nevocellular nevus. Plast Reconstr Surg. 1981; 67: 782-790. PubMed
© 2006 Dermatology Online Journal