Unilateral lower leg purpura
Published Web Location
https://doi.org/10.5070/D324c836brMain Content
Unilateral lower leg purpura
Satoshi Ogawa MD, Masahiro Oka MD PhD, Makoto Kunisada MD PhD, Chikako Nishigori MD PhD
Dermatology Online Journal 19 (1): 16
Kobe University Graduate School of Medicine Kobe, Hyogo, JapanAbstract
We present a case of an extensive, purpuric eruption on the lower leg with peculiar clinical findings in 55-year-old woman with rheumatoid arthritis. The purpuric lesions were present unilaterally on the left lower leg, where prominent varices and telangiectasia were noted. Histological examination revealed a perivascular infiltration of lymphocytic cells and eosinophils and extravasation of erythrocytes in the upper and middle dermis. There was no evidence of vasculitis. The eruption responded well to treatment with hemostatic agents and elastic stockings. Based on the clinical and histological findings, we concluded that the main pathophysiology of the purpuric eruption is an extravasation of erythrocytes related to increased venous pressure secondary to venous stasis.
The appearance of an extensive unilateral purpuric eruption on the lower leg is unusual. Herein, we describe a case of an acute onset, unilateral purpuric eruption on the lower leg in a middle-aged woman with rheumatoid arthritis.
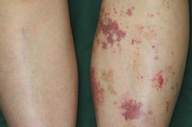
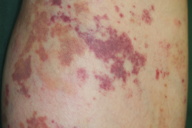
A 55-year-old woman with a past history of rheumatoid arthritis and left lower limb varices presented with 3-day history of an asymptomatic, violaceous eruption on the left lower leg. Initially, the eruption appeared as small macules adjacent to the varices on the medial aspect of the left popliteal fossa, but many similar macules subsequently developed over her left leg during the next 3 days. She had a 2-year history of rheumatoid arthritis and had been being treated with oral methotrexate (6-8 mg/week) and intravenous tocilizumab (200-480 mg/month) for 16 and 11 months, respectively, at the Department of Clinical Immunology of Kobe University Hospital. She had a 20-year history of varices on the medial aspect of her left lower leg, which had been managed conservatively by a cardiovascular surgeon. She had not done strenuous exercise prior to appearance of the eruption. Her arthralgias had not worsened recently.
On physical examination, she was afebrile and well. Multiple, irregularly shaped, brown macules and violet, non-palpable purpuric macules, less 4.0 cm in diameter were present on her left lower leg. Many punctate reddish-brown macules were also observed. The right leg, where there were no varices, did not show any lesions.
Laboratory findings were as follows: leukocyte count 3800/mm³ (normal 4000-8500/mm³), neutrophils 75.1 percent (normal 40-71%), stab 2 percent (normal 2-13%), seg 77.0 percent (normal 38-58%), eosinophils 8.4 percent (normal 0-7%), basophils 0.3 percent (normal 0-1%), monocytes 1.3 percent (normal 2-8%), lymphocytes 14.9 percent (normal 26-47%), red blood cell count 435 × 104/mm³ (normal 380-480 × 104/mm³), hemoglobin 12.4 g/dl (normal 11.8-15 g/dl), platelet 19.4 × 104/mm³ (normal 13-30 × 104/mm³), C-reactive protein <0.1 mg/dl (normal 0-0.3 mg/dl), aspartate amino transferase 35 IU/l (normal 13-31 IU/l), alanine aminotransferase 23 IU/l (normal 8-34 IU/l), γ-glutamic transferase 25 IU/l (normal 9-57 IU/l), alkaline phosphatase 222 IU/l (normal 109-321 IU/l), lactate dehydrogenase 252 IU/l (normal 115-217 IU/l), blood urea nitrogen 19 mg/dl (normal 9-22 mg/dl), creatinine 0.69 mg/dl (normal 0.5-1.3 mg/dl), IgG 2500 mg/dl (normal 870-1700 mg/dl), IgA 232 mg/dl (normal 110-410 mg/dl), and IgM 234 mg/dl (normal 46-260 mg/dl). Proteinuria and microscopic hematuria were not found.
 |  |
| Figure 1 | Figure 2 |
|---|
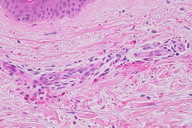
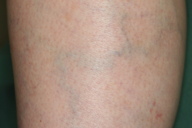
Although a definitive diagnosis was not made, the eruption was thought to be unrelated to systemic disease. The patient was treated with oral carbazochrome sodium sulfonate (90 mg/day) and tranexamic acid (750 mg/day). The skin eruption promptly resolved within 6 days and oral therapy was discontinued. However, similar lesions reappeared associated with pruritus in the subsequent week (Figures 1 and 2). Histological examination of a skin biopsy taken from a red-violaceous area of purpura revealed a normal epidermis and a perivascular infiltration of lymphocytic cells and eosinophils and prominent extravasation of erythrocytes in the upper and middle dermis (Figure 3). Interstitial infiltration of eosinophils was seen in the collagen bundles in the upper and middle dermis (Figure 3). There was no evidence of vasculitis. Direct immunofluorescence was positive for C1q and C3 in the deep dermis. Treatment with oral carbazochrome sodium sulfonate and tranexamic acid was restarted on the day of biopsy. In addition, an elastic stocking for the varices was applied. Most of the skin lesions, except for those near the ankle, resolved within 9 days after restarting treatment, leaving faint brown macules (Figure 4). Prominent venous dilatation and teleangiectasia were noted in the area where the purpuric eruption had been previously (Figure 5). Oral treatment was continued for 7 weeks. A small number of tiny purpuric macules less than 5 mm in diameter continued to intermittently appear and disappear in the 1 month after the biopsy. At 6 months follow-up, there was no sign of recurrence.
 |  |
| Figure 3 | Figure 4 |
|---|
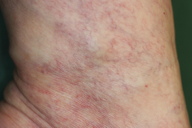 |
| Figure 5 |
|---|
We believe that the main pathophysiology of the purpuric eruption is an extravasation of erythrocytes related to increased venous pressure secondary to venous stasis. This hypothesis is supported by the following observations: prominent teleangiectasia was present on the lower leg, the dorsum of the left foot was spared (where the venous pressure is not so high), and hemostatic agents and elastic stockings were very effective for the treatment and prophylaxis of the purpuric eruption.
Although rare, unilateral lower leg purpuric eruption has been reported as a manifestation of Schamberg disease [1, 2], one of the four skin diseases known as “pigmented purpuric dermatoses” [3, 4]. There are distinct clinical differences between the present case and Schamberg disease. Firstly, the present case had an acute onset and purpuric lesions appeared extensively over the lower leg within a few days, resulting in the presence of both new and old lesions at the same time. These included both fresh purpura and their resolved lesions (purpura and brown macules, respectively). In contrast, the eruption of Schamberg disease initially appears as punctate macules, which slowly become a brown pigmentation. Purpura is not seen in Schamberg disease [3, 4]. Secondly, the recurrence of the eruption was associated with pruritus, whereas Schamberg disease is not generally associated with this symptom. Thirdly, the eruption responded promptly to hemostatic agents and elastic stockings without residual eruptions, whereas Schamberg disease is usually chronic, therapy-resistant, and persistent for years. Fourthly, our patient is 55 years of age, but unilateral Schamberg disease was described in young peoplemm³ [1, 2].
Our patient with rheumatoid arthritis had been treated with methotrexate and tocilizumab. Although rheumatoid arthritis has many skin manifestations, the skin eruption seen in the present case has not been described previously [5, 6]. To the best of our knowledge, we are unaware of methotrexate- or tocilizumab-induced drug eruption manifesting as a purpuric eruption. Thus, the skin eruption in the present case appears to be unrelated to rheumatoid arthritis or medications. However, we cannot exclude the possibility that a vascular abnormality caused by rheumatoid arthritis and/or medications used for its treatment, together with the preexisting varices, might be involved in the etiology of the skin eruption.
References
1. Hersh CS, Shwayder TA. Unilateral progressive pigmentary purpura (Schamberg's disease) in a 15-year-old boy. J Am Acad Dermatol. 1991 Apr;24(4):651. [PubMed]2. Nagata K, Danno K, Tanaka S. Unilateral Schamberg disease in a 14-year-old Japanese boy. J Dermatol. 1999 Jun;26(6):348-51. [PubMed]
3. Schroeder T. Pigmented purpuric dermatoses. In: Fitzpatrick TB, Eisen AZ, Wolff K, Austen KF, Goldsmith LA, Katz SI, eds. Dermatology in General Medicine. 6th ed. New York: McGraw-Hill, 2003: 1735-9.
4. Sardana K, Sarkar R, Sehgal VN. Pigmented purpuric dermatoses: an overview. Int J Dermatol. 2004 Jul;43(7):482-8. [PubMed]
5. Yamamoto T. Cutaneous manifestations associated with rheumatoid arthritis Rheumatol Int. 2009 Jul;29(9):979-88. [PubMed]
6. Clarke JT, Werth VP. Rheumatic manifestations of skin disease. Curr Opin Rheumatol. 2010 Jan;22(1):78-84. [PubMed]
© 2013 Dermatology Online Journal

