Facial angiofibromas in a mosaic pattern tuberous sclerosis: A case report
Published Web Location
https://doi.org/10.5070/D32005v8w3Main Content
Facial angiofibromas in a mosaic pattern tuberous sclerosis: A case report
Jasem M Alshaiji, Christopher R Spock, Elizabeth A Connelly, Lawrence A Schachner
Dermatology Online Journal 18 (7): 8
Department of Dermatology & Cutaneous Surgery, University of Miami - Miller School of Medicine, Miami, FloridaAbstract
Tuberous sclerosis is a rare genetic disorder presenting clinically with multiple hamartomas in different organs including the skin. The cutaneous manifestations include facial angiofibromas, hypopigmented macules (ash leaves), connective tissue nevi (shagreen patches), and periungual fibromas (Koenen tumors). We present a case of facial angiofibromas in a mosaic pattern tuberous sclerosis in an 11-year-old boy.
Introduction
Tuberous sclerosis complex (TSC) is a rare genetic neurocutaneous disorder that occurs in 1/6000 live births. It is inherited in an autosomal dominant pattern and it is characterized by the presence of hamartomatous growths in multiple organs, notably the brain, skin, eyes, kidneys, heart, and lungs. Two mutated genes are responsible for TSC, either TSC1, which encodes hamartin and is located on chromosome 9 (q arm), or TSC2, which encodes tuberin and is located on chromosome 16 (p arm). Both hamartin & tuberin form a complex that normally inhibits the mammalian target of rapamycin (mTOR) in vivo. Mutations in TSC1 or TSC2 result in defects in mTOR signaling inhibition leading to abnormal cellular proliferation, which occurs in the TSC-related hamartomas. TSC2 mutations occur more commonly (66-70% of the cases) and generate a more severe phenotype compared to TSC1 mutations. A mosaic pattern of tuberous sclerosis, characterized by the presence of facial angiofibromas with unilateral facial distribution, has been previously reported [1]. We report a case of facial angiofibromas in a mosaic pattern TS in an 11-year old boy.
Case report
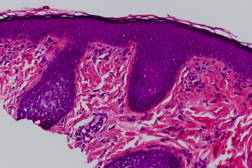
An 11-year-old boy presented to our University hospital complaining of asymptomatic progressive facial bumps for two years. He was diagnosed as having acne vulgaris in the past and was prescribed topical antiobiotics. However, no improvement was noticed. Eventually, he underwent skin biopsy for the facial lesions and the diagnosis of angiofibromas was made (Figure 3). The patient denies history of seizures or any other medical conditions. He had a long history of atopic eczema managed with topical steroids as needed for flares. There was no family history of tuberous sclerosis. Mental and developmental milestones were normal.
 |
| Figure 3 |
|---|
| Figure 3. Paucicellular fibrous proliferation associated with dilated, thin-walled vessels and stellate fibroblasts |
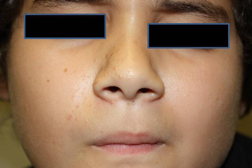
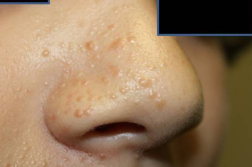
Full body skin exam was performed and revealed no abnormalities except scattered skin colored to brown 1-2 mm papules on the right nasolabial fold and right nasal side wall (Figures 1 and 2), a red excoriated plaque on the left antecubital fossa, telangiectasias over the dorsal aspect of left hand, and a firm, freely mobile, subcutaneous nodule in the right axilla. No ash leaf spots or confetti macules were observed under wood’s lamp evaluation; no collagenomas or koenen tumors were found. Because there were no other signs of tuberous sclerosis other than right-sided facial angiofibromas, a diagnosis of a mosaic pattern of tuberous sclerosis was made. The patient was referred to genodermatosis clinic for further evaluation and management.
Discussion
Tuberous sclerosis is an autosomal dominant disorder. Sporadic cases are estimated to constitute between 50 percent and 75 percent of all cases [1]. TSC was first recognized by Friedrich Daniel von Recklinghausen in 1862. The term tuberous sclerosis was coined in 1880 by Bourneville. In 1908, Vogt elucidated the classic diagnostic triad of intractable epilepsy, mental retardation, and facial angiofibromas (formerly termed adenoma sebaceum) [2, 3]. The clinical presentation of the disease varies from adults with very few signs of the disease to children with severe neurological involvement [4]. Tuberous sclerosis is most commonly associated with neurologic morbidity (epilepsy, cognitive impairment, autism, and behavioral disorders) occurring in up to 90 percent of patients. Renal manifestations are the next most common (angiomyolipomas and polycystic kidney disease) as well as skin lesions, occurring in up to 80 percent of patients. Less common are retinal hamartomas in up to 50 percent of patients; lung disease (lymphangioleiomyomatosis) is uncommon but potentially fatal [3, 5]. The most frequent cutaneous findings in TS include multiple facial angiofibromas, hypopigmented macules, periungual fibromas (Koenen tumors), and shagreen patches [1]. Major and minor criteria exist to diagnose tuberous sclerosis. The diagnosis is made when two major features, or one major and two minor ones, can be shown [5]. The presence of facial angiofibromas (70%-80% of cases) is a major criterion and is pathognomic of TS [1]. Because of the late onset of many signs and symptoms of TSC, molecular genetic testing (e.g. PCR and DNA sequencing) facilitates the confirmation of the diagnosis (75%-80% of the cases) by identification of the mutated TSC1/TSC2 genes in younger patients and even the prenatal or pre-implantation diagnosis can be established [2, 6]. Early recognition of TS is vital because prompt implementation of the recommended diagnostic evaluation may prevent serious clinical consequences (e.g., neurological manifestations) [2]. Unilateral multiple facial angiofibromas may form part of the clinical spectrum of TS probably as a consequence of cutaneous mosaicism in which postzygotic genetic mutation has occurred. In all previous publications the possibility of having minor or abortive TS was considered [1].
Treatment of angiofibromas is challenging. Therapeutic options reported include cryosurgery, curettage, dermabrasion, chemical peels, excision, laser therapy (e.g. pulse dye laser-PDL or ablative fractional resurfacing-AFR), electrosurgery, podophyllin, 5-aminolevulinic acid photodynamic therapy (PDT), and different combination modalities (pinpoint electrosurgery, PDL and AFR or PDT followed by PDL). A variety of degrees of success and side effects have been reported [7, 8, 9]. Recently, rapamycin (sirolimus), either topical or systemic, has been proposed for the treatment of cutaneous angiofibromas [10, 11, 12, 13].
References
1. Silvestre JF, Banuls J et al. Unilateral multiple facial angiofibromas: A mosaic form of tuberous sclerosis. J Am Acad Dermatol 2000;43(1):127-129. [PubMed]2. Schwartz R, Fernandez G et al. Tuberous sclerosis complex: Advances in diagnosis, genetics, and management. J Am Acad Dermatol. 2007; 57(2):189-202. [PubMed]
3. Krueger DA, Franz DN. Current management of tuberous sclerosis complex. Pediatr Drugs 2008;10(5):299-313. [PubMed]
4. Cabrera Lopez C, Marti T et al. Effects of rapamycin on angiomyolipomas in patients with tuberous sclerosis. Nefrologia 2011;31(3):292-8. [PubMed]
5. Curatolo P, Bombardieri R, Jozwiak S. Tuberous sclerosis. Lancet 2008;372:657-68. [PubMed]
6. Tarasewicz A, Debska-Slizien A et al. Rapamycin as a Therapy of Choice after renal transplantation in a patient with tuberous sclerosis complex. Tranplantation Proceedings 2009;41,3677-3682. [PubMed]
7. Weinberger CH, Endrizzi B et al. Treatment of angiofibromas of tuberous sclerosis with 5-aminolevulinic acid blue light photodynamic therapy followed by immediate pulsed dye laser. Dermatol Surg 2009;35:1849-1851. [PubMed]
8. Weiss ET, Geronemus RG. New technique using combined pulsed dye laser and fractional resurfacing for treating facial angiofibromas in tuberous sclerosis. Lasers in Surgery and Medicine 2010; 42:357-360. [PubMed]
9. Hofbauer GFL, Marcollo-Pini A et al. The mTOR inhibitor rapamycin significantly improves facial angiofibroma lesions in a patient with tuberous sclerosis. Br J Dermatol 2008;159:473-475. [PubMed]
10. Dabora SL, Franz DN et al. Multicenter Phase 2 Trial of Sirolimus for Tuberous Sclerosis: Kidney Angiomyolipomas and Other Tumors Regress and VEGF-D Levels Decrease. Multicenter Sirolimus Trial for Tuberous Sclerosis. PloS One 6(9): e233379. [PubMed]
11. Salido R, Garnacho-Saucedo G et al. Sustained clinical effectiveness and favorable safety profile of topical sirolimus for tuberous sclerosis-associated facial angiofibroma. J Eur Acad Dermatol Ven 2011. [PubMed]
12. Haemel AK, O'Brian AL et al. Topical rapamycin: A novel approach to facial angiofibromas in tuberous sclerosis. Arch Dermatol 2010;146(7):715-718. [PubMed]
13. DeKlotz CMC, Ogram AE et al. Dramatic improvement of facial angiofibromas in tuberous sclerosis with topical rapamycin: Optimizing a treatment protocol. Arch Dermatol 2011;147(9):1116-1117. [PubMed]
© 2012 Dermatology Online Journal