A case of infliximab-induced psoriasis
Published Web Location
https://doi.org/10.5070/D31zh0d26qMain Content
A case of infliximab-induced psoriasis
AG Richetta, C Mattozzi, V Carlomagno, E Maiani, V Carboni, S Giancristoforo, S D'Epiro, F Bruni, S Calvieri
Dermatology Online Journal 14 (11): 9
Department of Cutaneous and Venereal Diseases and Plastic Surgery, Policlinico Umberto I, University of Rome "La Sapienza,"
Rome, Italy. antoniorichetta@hotmail.comAbstract
Anti-tumor necrosis factor (anti-TNF-α) are a group of new drugs able to inhibit the action of this cytokine. Although systemic side effects have been well described, cutaneous adverse reactions have not yet been clearly elucidated. The authors report a case of a 29-year-old man affected by Crohn disease and ankylosing spondylitis who developed psoriatic lesions after IV infusion of infliximab 5 mg/Kg. The patient underwent cyclosporine treatment after interruption of biological therapy, and had complete resolution of cutaneous lesions. The reason for this phenomenon is not clear, Obviously more studies are necessary to define more clearly this paradoxical reaction. In addition, dermatologists must be informed about this potential cutaneous adverse event.
Introduction
Anti-tumor necrosis factors (Anti-TNF)-α are a group of new drugs able to inhibit the action of this cytokine, modulate the inflammation, and improve diseases in which TNF-α is mainly involved. The drugs in this group include monoclonal antibodies, infliximab and adalimumab, and the soluble TNF-α receptor, etanercept. These drugs have been approved for treatment of Crohn disease, rheumatoid arthritis (RA), ankylosing spondylitis, psoriatic arthritis, and psoriasis. Even if clinical trials have shown the safety of these drugs, some side effects have been described. Injection site reactions and increased risk of infection (in particular, reactivation of tuberculosis) are associated with the use of these agents. Increased risk of lymphoproliferative disease, the development of lupus-like syndromes and demyelination, including optic neuritis and reactivation of multiple sclerosis, are under evaluation in long-term follow-up studies [1]. Despite the fact that systemic side effects have been well described, cutaneous adverse reactions have not yet been clearly elucidated. For this reason it is necessary to have dermatological management of patients for biological treatments. Dermatologists must be informed of potential adverse cutaneous manifestations. A variety of skin eruptions (new onset or aggravations) have been described in patients with rheumatic disease during treatment with TNF-α antagonists; chronic inflammatory skin diseases, such as psoriasis and eczema-like manifestations, represent the majority of cases. Cutaneous infections caused by viral, bacterial, and fungal agents have been observed in many patients. Skin diseases such as dermatitis herpetiformis, leucocytoclastic vasculitis, and alopecia areata have occured in a few cases [2]. Other studies describe cutaneous reactions such as delayed skin eruption, lupus-like syndrome, cutaneous vasculitis, palmoplantar pustulosis, psoriasis vulgaris, atopic dermatitis, lichenoid rash, purpuric capillaritis, and melanoma [3]. Cases of interstitial granulomatous dermatitis, erythema multiforme, urticaria and angioedema-like skin eruptions have been reported as reactions to anti-TNF-α therapy [4, 5, 6].
In this report we describe a case of diffuse psoriasis in a patient treated with infliximab for Crohn disease and ankylosing spondylitis.
Case report
 |  |
| Figure 1 | Figure 2 |
|---|
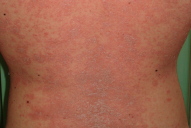
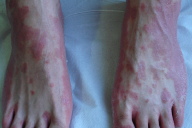
A 29-year-old man was admitted to our department for the spreading of a pruritic erythematous papulosquamous eruption on his trunk, buttocks, arms and legs. Futhermore he developed hyperkeratotic erythematous lesions on the palmar and plantar surface of his hands and feet and erythematous scaly lesions on the scalp (Figs. 1 & 2). He was on treatment with infliximab (5 mg/Kg body weight) for Crohn disease and ankylosing spondylitis, with the following regimen: at weeks 0, 2, 6 and then every 8 weeks. He developed the reactions after the fourth infusion at week 14 after the beginning of the treatment.
He had no personal and familiar history of psoriasis, skin rash or allergic reactions. Hematochemical assays revealed relative neutrophilia 76.8 percent (40-74) and lymphopoenia 17.2 percent (19-48). Anti-infliximab antibody was not detected. Cultures of swab samples taken from the pharynx grew Staphylococcus aureus, Klebsiella oxytoca and Streptococcus agalactiae; for this reason he started therapy with cyprofloxacin 500 mg twice a day for 7 days.
 |  |
| Figure 3a | Figure 3b |
|---|
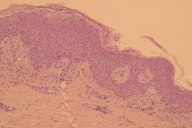
Skin biopsy showed psoriatiform dermatitis with acanthotic and para-keratotic epidermis, hyperplasia of epidermis, subcorneal micropustules filled with neutrophils, papillomatosis, focal absence of granular layer, spongiosis, and lymphocytic exocytosis. In the upper dermis were noted dilated capillaries, surrounded by a lymphohistocytic cellular infiltrate (Figs. 3a & 3b). This condition was consistent with psoriasis.
We decided to interrupt the infliximab infusions and to begin treatment with oral cyclosporine at the dosage of 250 mg/day for one month. The dose was progressively reduced down to a dose of just 50 mg a day. A complete resolution of the cutaneous eruption was obtained. After six months of follow up our patient had no cutaneous lesions. After six months of follow up patient did not present any cutaneous lesions.
Discussion
Tumor necrosis factor-α is one of the most important cytokines implicated in the development of rheumatic and inflammatory diseases, such as rheumatoid arthritis, ankylosing spondilitis, psoriasic arthritis, and psoriasis. It is possible to modulate the inflammation just by blocking the action of this molecule; improvement is seen in a large number of diseases in which this cytokine plays a pivotal role. The inflammatory response in psoriatic plaques is initiated in part by activated T cells in the epidermis and dermis. Th1 T cells are predominant in plaques and play an important role in the pathogenesis of this disease; they release inflammatory cytokines including TNF-α. Levels of TNF-α, γ-interferon, interleukin (IL)-6, IL-8, and IL-12, are increased in psoriatic lesions along with activation of some trascription factors such as NFκB. Futhermore TNF-α may also further elevate levels of these same cytokines [7]. The action of tumor necrosis factor-α is prominant on antigen presenting cells (APC) of skin (Langerhan cells); it stimulates these cells to migrate from skin to lymph node where antigen is presented to T cells. The interaction of MHC class I or II bound antigens with T cell receptor (TCR), with a second activating signal, causes the activation of T lymphocytes that express cutaneous lymphocyte-associated antigen (CLA). This activation allows them to migrate into the dermis. The E-cadherin decrease in expression on the Langerhan cell surface facilitates migration into the dermis by decreasing interactions with keratinocytes [8]. TNF-α plays an important role on endothelial cells by increasing expression of adhesion molecules and stimulating the production of angiogenic factors such as vascular endothelial growth factor (VEGF) [9, 10, 11]. Anti-TNF-α drugs are able to reduce other inflammatory cytokines such as IL-6, IL-8, CSF, and VEGF. Inhibiting the action of TNF-α reduces the inflammatory process and the proliferation of keratinocytes and endothelial cells [12, 13]. That is the reason why these drugs are used to treat moderate to severe psoriasis that is not responding to common therapies. For these reason psoriasis induced by an anti-TNF-α may seem paradoxical.
In the literature several cases of psoriasis associated with biological drugs have been described [14, 15, 16, 17]. This reaction was more frequent in women and was often in patients without a personal history of psoriasis. There is a predilection for palm and sole involvement. Patients rapidly improve after discontinuing anti-TNF-α treatment along with the initiation of other psoriasis treatments. Even if in some cases it was possible to continue the treatment, it is not clear if this is the best solution or if it would be preferable to change to another anti-TNF-α inhibitor [18].
The reason for this phenomenon is not clear. Infliximab may enhance susceptibility to bacterial infections caused by TNF-α inhibition; this condition is revealed in our patient by the positivity of bacterial cultures by pharingeal swab. Chronic infections with various agents as well as superantigens released might activate T cells, followed by the induction of psoriatic lesions. It is possible that some of these patient could be afflicted with psoriatic arthritis, not with ankylosing spondilitis, and the new onset of psoriatic lesions could be a normal evolution of this disease.
Biological therapy may alterate the normal cytokinic pattern, modifying the normal balance between different molecules. This could result in a relative increase of INF-γ that would be consistant with development of psoriatic lesions, due to an excessive activation of lymphocytes.
As described in the literature TNF-α may have a suppressive regulatory role, preventing the activation of psoriasis specific autoreactive T cells, in particular cutaneous lymphocyte antigen (CLA) [19]. Furthermore tumor necrosis factor-α may regulate the expression of some chemokine receptors, such as CXCR3 that promote infiltration of T cells to the skin; this chemokine is overexpressed in psoriasic lesions and anti-TNF treatment may increase levels of this molecule, favoring the development of psoriasis [20, 21].
To our knowledge the reason for this cutaneous side effect is not clear and will require further study to determin the cause for this paradoxical reaction, but dermatologists should be aware of this cutaneous adverse reaction.
References
1. Nash PT, Florin THJ. Tumour necrosis factor inhibitors. MJA 2005; 183(4): 205-208. PubMed.2. Lee HH, Song IH, Friedrich M, Gauliard A, Detert J, Rowert J, Audring H, Kary S, Burmester GR, Sterry W, Worm M. Cutaneous side-effects in patients with rheumatic disease during application of tumor necrosis factor-alpha antagonists. Br J Dermatol 2007 Mar; 156(3): 486-91. PubMed.
3. Lebas D, Staumont-Sallé D, Solau-Gervais E, Flipo RM, Delaporte E. Cutaneous manifestations during treatment with TNF-alpha blockers: 11 cases. Ann Dermatol Venereol 2007 Apr; 134(4 Pt 1): 337-42. PubMed.
4. Beuthien W, Mellinghoff HU, Von Kempis J. Skin reaction to adalimumab. Arthritis Rheum. 2004 May; 50(5): 1690-2. PubMed.
5. Devos SA, Van Den Bossche N, De Vos M, Naeyaert JM. Adverse skin reactions to anti-TNF-alpha monoclonal antibody therapy. Dermatology. 2003; 206(4): 388-90. PubMed.
6. Nikas SN, Voulgari PV, Drosos AA. Urticaria and angiedema-like skin reactions in a patient treated with adalimumab. Clin Rheumatol. 2007 May; 26(5): 787-8. PubMed.
7. O'Shea JJ, Ma A, Lipsky P. Cytokines and autoimmunity. Nat Rev Immunol 2002;2:37-45. PubMed .
8. Kimber I, Cumberbatch M, Dearman RJ, Bhushan M, Griffiths CEM. Cytokines and chemokines in the initiation and regulation of epidermal Langerhans cell mobilization. Br J Dermatol 2000;142:401-12. PubMed.
9. Gottlieb AB, Krueger JG, Wittkowski K, Dedrick R, Walicke PA, Garovoy M. Psoriasis as a model for T-cell-mediated disease: Immunobiologic and clinical effects of treatment with multiple doses of efalizumab, an anti-CD11a monoclonal antibody. Arch Derm 2002;138:591-600. PubMed.
10. Bhushan M, McLaughlin B, Weiss JB, Griffiths CE. Levels of endothelial cell stimulating angiogenesis factor and vascular endothelial growth factor are elevated in psoriasis. Br J Dermatol. 1999 Dec;141(6):1054-60. PubMed
11. Simonetti O, Lucarini G, Goteri G, Zizzi A, Biagini G, Lo Muzio L, Offidani A. VEGF is likely a key factor in the link between inflammation and angiogenesis in psoriasis: result of an immunohistochemical study. Int J Immunopathol Pharmacol. 2006 Oct-Dec;19(4):751-60. PubMed.
12. Mease P. TNF-α therapy in psoriasis arthritis and psoriasis. Ann Rheum Dis 2004; 63: 755-758. PubMed.
13. Cordiali-Fei P, Trento E, D'Agosto G, Bordignon V, Mussi A, Ardigo M, Mastroianni A, Vento A, Solivetti F, Berardesca E, Ensoli F. Decresed levels of metalloproteinase-9 and angiogenic factors in skin lesions of patients with psoriasic arthritis after therapy with anti-TNF-alpha. J Autoimmune Dis. 2006 Oct 5;3:5. PubMed.
14. P. P. Sfikakis, A. Iliopoulos, A. Elezoglou, C. Kittas, and A. Stratigos. Psoriasis Induced by Anti-Tumor Necrosis Factor Therapy: A Paradoxical Adverse Reaction. Arthritis Rheum. 2005 Aug; 52(8): 2513-2518. PubMed.
15. Takahashi H, Hashimoto Y, Ishida-Yamamoto A, Ashida T, Kohogo Y, Iizuka H. Psoriasiform and pustular eruption induced by infliximab. Journ Dermatol. 2007; 34: 468-472. PubMed.
16. Kary S, Worm M, Audring H, Huscher D, Renelt M, Sorensen H, Stander E, Maaß U, Lee H, Sterry W, Burmester GR. New onset or exacerbation of psoriatic skin lesions in patients with definite rheumatoid arthritis receiving tumour necrosis factor-α antagonists. Ann Rheum Dis 2006;65: 405-407. PubMed.
17. Severs GA, Lawlor TH, Purcell SM, Adler DJ, Thompson R. Cutaneous adverse reaction to infliximab: report of psoriasis developing in 3 patients. Cutis 2007 Sep; 80(3): 231-7. PubMed.
18. Heymann WR. Tumor necrosis factor inhibitor-induced pustular psoriasis? J Am Acad Dermatol. 2007 ; 56(2): 327-328. PubMed.
19. Berg EL, Yoshino T, Rott LS, Robinson MK, Warnock RA, Kishimoto TK, et al. The cutaneous lymphocyte antigen is a skin lymphocyte homing receptor for the vascular lectin endothelial cell-leukocyte adhesion molecule 1. J Exp Med 1991;174:1461-6. PubMed.
20. Aeberli D, Seitz M, Juni P, Villiger PM. Increase of peripheral CXCR3 positive T lymphocytes upon treatment of RA patients with TNF-α inhibitors. Rheumatology (Oxford) 2005;44:172-5. PubMed.
21. Rottman JB, Smith TL, Ganley KG, Kikuchi T, Krueger JG. Potential role of the chemokine receptors CXCR3, CCR4, and the integrin _E_7 in the pathogenesis of psoriasis vulgaris. Lab Invest 2001;81:335-47. PubMed.
© 2008 Dermatology Online Journal

