Sneddon-Wilkinson disease resistant to dapsone and colchicine successfully controlled with PUVA
Published Web Location
https://doi.org/10.5070/D31vn304vnMain Content
Sneddon-Wilkinson disease resistant to dapsone and colchicine successfully controlled with PUVA
Amor Khachemoune MD and Marianna L Blyumin BS
Dermatology Online Journal 9 (5): 24
Division of Dermatology, Georgetown University Medical Center, Washington DC. Amorkh@pol.net
Abstract
A 28-year-old man with a 6-year history of subcorneal pustular dermatosis (SPD) is presented. This case reviews the clinical features, differential diagnosis, etiology, and treatment options of SPD. In particular, this case emphasizes the absence of response to dapsone and colchicine and control with PUVA therapy.
Clinical summary
History.—A 28-year-old Caucasian man presented with 6-year duration of recurrent, erythematous, crusted, asymptomatic papules, scaly plaques, and grouped pustules affecting the neck, trunk, flexural aspects of extremities, and the groin. Review of systems was unremarkable and his family and social histories were noncontributory.
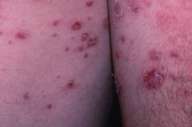
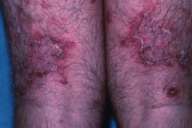
Physical examination.—On initial presentation, there were multiple pustules and papules as well as annular and serpiginous pink plaques with central scales and crusts and surrounding deep erythema. These lesions were symmetrically distributed on the groin and proximal upper and lower extremity flexor aspects (figs. 1 and 2).
|
|
| Figure 1 | Figure 2 |
|---|
Laboratory data.—Laboratory investigation including complete blood count, liver function tests, serum and urine protein electrophoreses, and rheumatoid factor were all within normal limits.
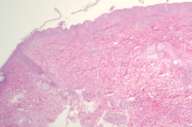
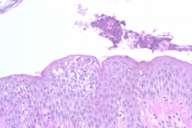
Histopathology.—A skin biopsy of a representative lesion was performed. Histology sections showed subcorneal separation with focal aggregates of keratin and neutrophils within the cleft. The epidermis showed mild and diffuse spongiosis with focal exocytosis of neutrophils without acantholysis. The upper dermis contained a patchy infiltrate composed of lymphocytes, histiocytes and neutrophils. On direct immunofluorescence IgA and IgM were negative. A periodic acid-Schiff stain for fungal organisms was negative. A Gram stain was negative for bacteria (figs. 3 and 4). Based on the histology, the diagnosis was determined to be Sneddon-Wilkinson syndrome (SWD) or Subcorneal pustular dermatosis (SPD).
|
|
| Figure 3 | Figure 4 |
|---|
Clinical course.—The patient was treated initially with oral dapsone (50 mg daily) for 3 months; then with a combination of daily dapsone (50 mg) and colchicine (0.6 mg) for another 3 months without a response. The treatment was discontinued after he experienced side effects of diarrhea and a subsequent 20-pound weight loss. Topical mid- to high-potency corticosteroids were then used for 2 months without effect. He was then started on PUVA therapy twice-a-week for 6 weeks, then once-a-week for 1 month, then once every other week for 2 additional months, then once-a-month thereafter. PUVA was controlling the disease until the patient experienced a mild flare on discontinuation 6 months later. He was then restarted on PUVA with significant improvement. He is currently receiving maintenance PUVA therapy once every 3 weeks, which is keeping the disease under good control.
Comment
SPD is a rare, chronic pustular dermatosis that was initially described by Sneddon and Wilkinson in 1956 [1]. It is more commonly seen in women then men, and is characterized by the asymptomatic eruption of grouped pustules that usually develop into half-pustular, half-clear fluid blisters and coalesce to form annular or circinate shapes. SPD usually appears symmetrically on the axillae, groin, abdominal folds, inframammary areas, and flexural aspects of the extremities. The evolution of the lesions commonly leads to scaling, crusting, and rarely results in mild hyperpigmentation. The key to diagnosing SPD is a biopsy demonstrating a sterile subcorneal pustule filled with neutrophils, absence of acantholysis, and negative immunofluorescence.
The main differential diagnosis of SPD includes subcorneal-type of IgA pemphigus, pemphigus foliaceus, dermatitis herpetiformis, pustular psoriasis, acute generalized pustulosis, and bacterial impetigo [2]. Subcorneal-type of IgA pemphigus is a cutaneous disorder that clinically resembles SPD, but differs in that immunofluorescence demonstrates positive intraepidermal IgA deposits directed against desmocollin-1 [3]. Its classification as a variant of pemphigus versus SPD is currently controversial [4]. In our patient, the immunofluorescence stains were negative, ruling out the diagnosis of subcorneal-type of IgA pemphigus. A diagnosis of pemphigus foliaceus was also unlikely because there was an absence of both acantholysis and epidermal intercellular IgG immunofluorescence. Dermatitis herpetiformis was ruled out because of the lack of pruritus, subepidermal vesicles, and granular-IgA in the dermal papilla. The absence of constitutional symptoms and the prominent spongiform changes on histology were inconsistent with a diagnosis of pustular psoriasis, despite the fact that PUVA therapy was an effective treatment. Acute generalized exanthematous pustulosis is characterized by the presence of systemic symptoms and histologic leukocytoclastic vasculitis, both absent in our patient. Finally, the absence of pathogenic organisms within the pustules ruled out bacterial and fungal infections.
Although the etiology of SPD is unknown, there are multiple theories including infectious or autoimmune mechanisms. SPD may be associated with disorders such as pyoderma gangrenosum, rheumatoid arthritis, systemic lupus erythematosus, inflammatory bowel diseases, hyperthyroidism, multiple myeloma, benign monoclonal IgA, IgG, and IgM gammopathy, and apudoma [2]. Due to the prominent relationship between SPD and autoimmune disorders, some authors have recommended to screen SPD patients for rheumatoid arthritis and monoclonal gammopathy [3].
The historical SPD's dramatic response to dapsone has led authors to not only use this medication as a first line treatment for this disease, but also to consider patient's response to this treatment a diagnostic marker [5]. However, SPD does not always respond to dapsone, as seen in our patient and other reported cases [6]. Although less effective, sulfapyridine, oral corticosteroids and retinoids, may be used as alternatives [7]. Anecdotal uses of colchicine, ketoconazole, minocycline, cyclosporine, mebhydroline, and infliximab also show potential for treating SPD [2, 7, 8, 9]; however, colchicine was ineffective in our patient. There are reports of UVB [10] and PUVA[11] efficacy in the management of SPD; this was likewise seen in our patient. Considering risks of malignancies following prolonged use of PUVA, and until a safer and cost-effective treatment for SPD is available, we will reduce the number of treatments to the minimum necessary to keep the disease under control.
Authors note: This work was presented at the Gross and Microscopic Symposium at the American Academy of Dermatology meeting in San Francisco, CA, March 2003.
References
1. Sneddon IB, Wilkinson DS. Subcorneal pustular dermatosis. Br J Dermatol 1956;68:385-394.2. Reed J, Wilkinson J. Subcorneal pustular dermatosis. Clin Dermatol 2000;18:301-313.
3. Scheinfeld NS, Worth R, Mallea J, Shookster L, Weinberg JM. Subcorneal Pustular Dermatosis Developing in a Patient With Rheumatoid Arthritis; Rheumatoid, Antimicrosomal, and Antimitochondrial Autoantibodies; and a Goiter. SKINmed 2003:2;258-259.
4. Yasuda H, Kobayashi H, Hashimoto T, Itoh K, Yamane M, Nakamura J. Subcorneal pustular dermatosis type of IgA pemphigus: demonstration of autoantibodies to desmocollin-1 and clinical review. Br J Dermatol 2000;143:144-148.
5. Lutz ME, Daoud MS, McEvoy MT: Subcorneal pustular dermatosis: a clinical study of ten patients. Cutis 1998;61:203-208.
6. Johnson SA, Cripps JC. Subcorneal pustular dermatosis in children. Arch Dermatol 1974;109:73-77.
7. Zachariae Co, Rossen K, Weismann K. An unusual severe case of subcorneal pustular dermatosis treated with cyclosporine and prednisone. Acta Derm Venereol 2000;80:386-387.
8. Dorittke P, Wassilew SW. Sneddon Wilkinson subcorneal pustulosis, therapy with mebhydroline. Z Hautkr 1988;63:1025-1027.
9. Voigtlaender C, Luftl M, Schuler G, Hertl M. Infliximab (anti-tumor necrosis factor alpha antibody): a novel, highly effective treatment of recalcitrant subcorneal pustular dermatosis (Sneddon-Wilkinson disease). Arch Dermatol 2001;137:1571- 1574.
10. Park YK, Park HY, Bang DS, Cho CK. Subcorneal pustular dermatosis treated with phototherapy. Int J Dermatol 1986;25:124-126.11. Bauwens M, De Coninck A, Roseeuw D. Subcorneal pustular dermatosis treated with PUVA therapy. A case report and review of the literature. Dermatology 1999;198:203-205.
© 2003 Dermatology Online Journal

