The Highly Effective Use of Topical Zinc Pyrithione in the Treatment of Psoriasis: A Case Report
Published Web Location
https://doi.org/10.5070/D31hr315zjMain Content
The Highly Effective Use of Topical Zinc Pyrithione in the Treatment of Psoriasis: A Case Report
Charles E. Crutchfield III, M.M.B., M.D., Eric J. Lewis, M.D., Ph.D., and Brian D. Zelickson, M.D
Dermatology Online Journal: 3(1) : 3
|
WARNING!
This product has been withdrawn from the American market by the U.S. Food and Drug Administration because of questions over ingredients and safety. (see the next issue). Parts of this article are taken verbatim from Crutchfield CE, Lewis EJ, Zelickson BD: The effective use of topical zinc pyrithione in the treatment of psoriasis: A report of three cases. J Geriatric Dermatol 5:21-24, 1997. This article should therefore considered a duplicate publication. AbstractPsoriasis is a common condition which, at times, can be difficult to treat. Patients with psoriasis face many social challenges and can suffer a great deal with their disease. As a result, any new topical agent is a welcomed addition to our therapeutic armamentarium. We present here a report of a case using a novel topical preparation of zinc pyrithione for the treatment of psoriasis. Topical zinc pyrithione appears to be a safe and effective treatment for psoriasis.
|
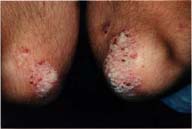
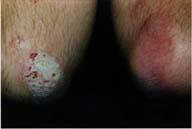
IntroductionPsoriasis affects approximately 20f the population. The clinical course is dynamic, capricious, and replete with flares and remissions.Currently there are many good anti-psoriatic treatment choices including topical agents (steroids, tars, anthrallin, and calcipotriene), phototherapeutics (UVB, PUVA), and systemic treatments (methotrexate, cyclosporine, and etretinate).(1,2,3) Often a combination of treatment modalities is required for clinical success. Unfortunately, phototherapeutic modalities can be labor intensive and inconvenient. Systemic treatments have significant side effect considerations. Consequently, any effective and safe topical agent is a welcomed addition to our therapeutic armamentarium. This report describes the use of a preparation containing zinc pyrithione (0.25%) in a vehicle containing isopropyl myristate to treat a case of psoriasis. We have recently become aware of a new anti-psoriatic treatment containing zinc pyrithione (which is also recognized as the active ingredient in a major anti-dandruff shampoo). The effectiveness of zinc pyrithione to treat seborrheic dermatitis and psoriasis has been well documented (4,5,6,7,8,13). Since some believe that seborrheic dermatitis and psoriasis may lie on different ends of the same spectrum, it seemed reasonable to try a topical preparation of zinc pyrithione to treat psoriasis. The mechanism of action of zinc pyrithione on psoriasis and seborrheic dermatitis has been reported to be anti-proliferative via "DNA interactions", anti-yeast, antiseptic, and keratinolytic mechanisms.(4,5,6,8) We report here the clinical results of a psoriatic patient treated with a topical spray containing zinc pyrithione. MethodsA 39 year old man with a 14 year history of moderate plaque psoriasis was instructed to spray the topical zinc pyrithione preparation, twice per day, to the left elbow. The right elbow was to be used as a control and received no treatment of any kind during the trial. The treatment preparation contains zinc pyrithione (0.25%) in a vehicle containing isopropyl myristate.ResultsFigure 1 depicts the baseline condition of the patient's elbows before trial initiation. Figure 2 depicts the patient's elbows 3 weeks after applying the zinc pyrithione preparation to the left elbow only. At the end of the 3 week treatment period the left (treated) elbow was essentially clear. The right (control) elbow remained virtually unchanged, if not slightly worse. The patient also reported a complete disappearance of pruritus approximately 3 days into treatment, on the zinc pyrithione preparation treated plaque only, that was sustained throughout the treatment period. |
|
| FIGURE 1 | FIGURE 2 |
|---|
|
Figure 1: Pretreatment (baseline). Figure 2: Three weeks of topical zinc pyrithione spray treatment to the patient's left elbow only. The patient's right elbow was untreated during the period.
|
DiscussionThe result in this case is impressive. There was marked reduction in scale, erythema, and pruritus. The patient reported no significant side effects during and after the treatment period. At a 6 week follow up the patient remained lesion free on the treatment plaque, with no additional applications. Perhaps a maintenance treatment plan (e.g. BID on weekends only), or the immediate re-treatment of any new/recurring plaque may reduce the frequency and/or severity of future flares.Although the exact mechanism of action of the "activated" zinc pyrithione preparation is unknown it may be speculated that the possible anti-proliferative mechanism of action of zinc pyrithione may involve the regulation of DNA transcription factors containing "zinc finger" binding domains.(9,10) It is also well recognized that many enzymes require the binding of metal ions for activation. Perhaps one of these (zinc requiring) enzymes or transcription factors plays a key role in the regulation of cellular proliferation. It is also well known that a deficiency of zinc produces a disease state (acrodermatitis enteropathica) that includes psoriasiform lesions (11,12). Perhaps the vehicle or the activated form of zinc pyrithione permits a physiologic level of zinc to be reached in the target cells, either epidermal and/or lymphocytic. The combination of zinc pyrithione with the other vehicle including isopropyl myristate may also have significant additional influence on clinical effectiveness.. In this preliminary report, an aerosol preparation of zinc pyrithione (0.25%) in a vehicle containing isopropyl myristate appears to be a safe and effective treatment for psoriasis. Each ml of the liquid preparation will cover an area the size of a palm, approximately 6-8 times. Because of the promising results in this case report and a plethora of anecdotal reports, we are initiating a 60 patient, double-blind, vehicle- controlled clinical study evaluating the use of topical zinc pyrithione for the treatment of psoriasis. If additional controlled research confirms these encouraging results, topical zinc pyrithione may represent one of the major advances n the treatment of psoriasis Conflict of Interest Statement:Our curent research is supported by an unrestricted educational grant from Cheminova International, S.A. Beyond this we have no other affiliations of conflicts of interest with the company or its products. The case presented here was funded entirely by the authors. Acknowledgments: We wish to acknowledge Drs. Mark Dahl, Cynthia Olson, Bruce Bart, Fred Fish, Steve Prawer, Humberto Gallego, Hector Gallego, Janellen Smith, Chris Zachary, Whitney Tope, Maria Hordinsky, Danielle Miller, Paul Lundstrom, Michelle Blaeser, Mark Pittelkow and all of the dermatology residents and faculty at the University of Minnesota for their encouragement, support, and comments on this project References1. Arndt, Le Boit, Robinson and Wintrub, [editors]. Cutaneous Medicine and Surgery. W.B. Sanders, & Co., Pubs. 1995. "Treatment of psoriasis", pages 304-316.2. Carruthers, R.: Psoriasis treatment options. Am Fam Physician. 21:1625-9,1992.3. 3. Phillips, TJ: Current treatment options in psoriasis. Hospital Practice. 31:155-166,1996. 4. Marks R, Pearse AD, Walker, AP: The effects of a shampoo containing zinc pyrithione on control of dandruff. Br J Dermatol 112:415-422,1985. 5. Shuster, S: The aetiology of dandruff and the mode of action of therapeutic agents. Br J Dermatol 111:235-242,1984. 6. Kligman AM, McGinley KJ, Leyden JJ: The nature of dandruff. J Soc Cosmet Chem 27:111-139,1976. 7. Arndt, Le Boit, Robinson, and Wintrub, [editors]. Cutaneous Medicine and Surgery. W.B. Saunders, & Co., Pubs. 1995. "Treatment of seborrheic dermatitis", page 217. 8. McGrath J, Murphy GM: The control of seborrheic dermatitis and dandruff by antipityosporal drugs. Drugs 41:178, 1991. 9. Bernstein BE, Hoffman RC, Klevit RE: Sequence-specific DNA recognition by Cys2,His2 zinc fingers. Ann NY Acad Sci. 726:92-104,1994. 10. Rhodes D, and Klug A: Zinc fingers. Sci. Am. 268:56-65,1993. 11. Graves, K., Kestenbaum T, and Kalivas J. Hereditary acrodermatitis enteropathica in an adult. Arch Dermatol 116:5, 562-564, 1980. 12. Neldner KH, Hambidge KM, and Walravens PA. Acrodermatitis enteropathica. Int J Dermatol 17:5, 380-387, 1978. 13. Final Monograph for dandruff, seborrheic dermatitis, and psoriasis over-the-counter drug products. Federal register. Washington, D.C.:U.S. Department of Health and Human Services, U.S. Food and Drug Administration: 56 FR 63554, Dec. 1991. |
© 1997 Dermatology Online Journal for use in electronic format, otherwise copyright is retained by the author(s) |

