Pseudoxanthoma elasticum-like syndrome and thalassemia: An update
Published Web Location
https://doi.org/10.5070/D31cw031gjMain Content
Pseudoxanthoma elasticum-like syndrome and thalassemia: An update
Elena Fabbri MD, Gian Luca Forni MD, Giulia Guerrini MD, Caterina Borgna-Pignatti MD
Dermatology Online Journal 15 (7): 7
Pediatrics, University of FerraraAbstract
Pseudoxanthoma Elasticum (PXE) is an autosomal recessive, multisystem disorder affecting connective tissues. We describe three cases of the acquired PXE-like syndrome that often occurs in association with hemolytic anemias, in particular the hemoglobinopathies, and review the literature on the subject. The pathogenesis of the acquired PXE-like lesions is not yet completely understood. None of the mutations observed in the inherited form has been detected in the syndrome accompanying thalassemia. The cardiovascular complications could be life-threatening. Therefore, an close surveillance of these patients is mandatory.
Introduction
In January 2009, Dermatology Online Journal published a review article on Pseudoxanthoma Elasticum (PXE) [1]. This is a rare, autosomal recessive, multisystem disorder affecting connective tissues. The genetic defect of inherited PXE has been mapped to the ABCC6 gene on chromosome 16p13.1 [2,3,4,5], which encodes a transmembrane transporter protein involved in cellular detoxification [6]. About 90 mutations have been identified [7]. It is characterized by a progressive degeneration and fragmentation of elastic fibers and presents with skin, ocular, and vascular lesions.
We wish to report on the acquired form of PXEthat has been described to complicate the course of several congenital hemolytic anemias. This elastopathy resembling PXE was firstly observed in sickle cell anemia in the late 1950s [8, 9] and subsequently, in sickle-thalassemia [10], beta-thalassemia major [11, 12] and minor [13, 14], spherocytosis [15], dyserithropoietic anemia type III [16], and other hemoglobinopathies [17, 18, 19, 20].
Case reports
Case 1. A 58-year-old male patient was diagnosed as being affected by non-transfusion dependent thalassemia intermedia at age 22. At that time he was splenectomized, received 5 U of red blood cells and became hepatitis B virus (HBV) positive. Ten years later, due to severe iron overload, he underwent 18 months of intensive iron-chelation therapy with desferrioxamine. The patient had been suffering from a retinopathy for the past 5 years. When he moved to our center his average Hb was 11.4 g/dl; platelets were 567.000/mmc; ferritin was 400 ng/ml. Bone densitometry demonstrated osteopenia. He was HBV antibody positive, HBs Ag negative, and hepatitis C virus (HCV) negative.
 |
| Figure 1 |
|---|
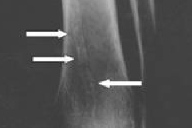
Shortly thereafter, an x-ray performed because of trauma to the left ankle showed diffuse fibrotic degeneration of the articular surface and evident calcification of the tibial vessels (Fig. 1). A Doppler sonography was then performed that revealed a bilateral severe atheromatous stenosis of the superficial femoral arteries and the presence of collateral circulation. Diffuse calcification of the abdominal aorta, the thoracic aorta, and some peripheral vessels were also present.
The consultant dermatologist identified several yellowish papules and plaques with a "plucked chicken" and "cobblestone" appearance in the left axillary area; these were biopsied.
 |  |
| Figure 2 | Figure 3 |
|---|
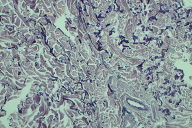
The skin biopsy confirmed the presence of histological PXE-like features with polymorphous fragmented elastic fibers and disorganized collagen bundles (Fig. 2).
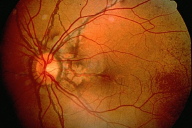
Bilateral angioid streaks were present in both fundi and subfoveal choroidal neovascularization with macular degeneration in the right eye (Fig. 3).
 |
| Figure 4 |
|---|
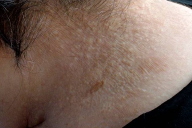
Case 2. A 46-year-old woman with homozygous sickle cell anemia presented with typical plaques of PXE on the neck (Fig. 4). A skin biopsy confirmed the clinical diagnosis. The patient had been transfused several times because of hemolytic and painful criss. She was HBV and HCV negative.
An echocardiography showed calcifications of the mitral valve.
Case 3. This is the case of a 30-year-old male with transfusion-dependent thalassemia diagnosed in the first year of life. Good control of iron overload (ferritin level 800 ng/ml) was obtained with regular chelation therapy.
 |  |
| Figure 5 | Figure 6 |
|---|
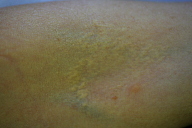
The diagnosis of PXE relied on the finding of small yellow xanthoma-like papules and plaques on the flexural skin of the neck and arm (Fig. 5). The skin appeared soft, lax and slightly wrinkled.
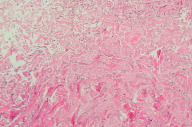
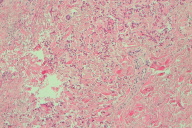
A biopsy showed the characteristic histological changes in the dermis.
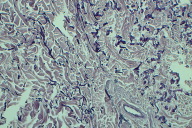
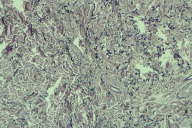
Sections of affected skin exhibited scattered focal fibrosis with an accumulation of many fragmented granular and calcified elastic fibers in the middle to deep dermis, interspersed between normal-appearing fiber bundles (Figs. 6 - 8).
 |  |
| Figure 7 | Figure 8 |
|---|
 |  |
| Figure 9 | Figure 10 |
|---|
 |
| Figure 11 |
|---|
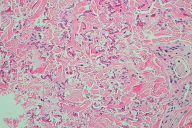
Fragmented granular material were stained black by the Weigert resorcin fuchsin method (Figs. 9 - 10).
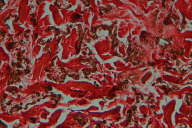
Degenerated elastho-collagen fibers were stained red by the Verhoeff method (Fig. 11).
Discussion
PXE and thalassemia
Thalassemia patients used to die in the first or second decades of life, but are now surviving longer. [21] Therefore, complications once unknown are being identified.
The coexistence of PXE and sickle-cell anemia was first described in 1964 [22]. The first report of two patients affected by thalassemia and coexistent PXE was published by Aessopos et al in 1989 [23]. Several subsequent reports have confirmed the existence of a clinical syndrome resembling PXE in thalassemia patients occurring with a frequency higher than in the general population [24, 25, 26, 27].
In a study published in 1998 and including patients affected by thalassemia intermedia older than 30 years of age, arterial calcifications were found in 55 percent, skin lesions in 20 percent, and ocular alterations in 52 percent. A total of 85 percent had at least one of the above three lesions [28].
The typical histopathological features of PXE, both in the inherited and the acquired form, are the abnormal, mineralized, and fragmented elastic fibers in skin, eyes and arterial blood vessels (elastorrhexia) [7, 29].
Several studies have investigated the relationship between these two conditions. In the PXE-like syndrome developing in thalassemia patients, the defect is believed to be acquired, although it shows a progression similar to that of the inherited form.
Cianciulli et al. [30] followed, for 12-years, a cohort of 80 Italian beta-thalassemia patients and showed an incidence of PXE-skin lesions of 17 percent (14/80). They reported that elastic fiber fragmentation and mineralization as well as collagen fibril alterations were identical to those described in the inherited form. These findings were in accordance with data [27] showing that PXE-like alterations in beta-thalassemia were indistinguishable from those of the inherited form.
None of the typical abnormalities was identifiable in the dermis of the parents of PXE-positive thalassemia patients, suggesting that inheritance of the PXE gene defect in these patients was highly unlikely. Furthermore, the research of an association between a specific haplotype and the appearance of the PXE syndrome in beta-thalassemia patients has been performed by Samarkos et al. [31], but no differences have been identified between the genotypes of PXE-positive thalassemia patients and those of the general beta-thalassemia population. The inherited PXE is caused by mutations in the ATP-binding cassette, subfamily C (CFTR/MRP), member 6 (ABCC6) gene. Because no disease-causing variant was found in the ABCC6 gene of 10 beta-thalassaemia patients with a PXE-like phenotype, a study suggested that the PXE-like symptoms in beta-thalassemia are not related to ABCC6 mutations [32].
In the acquired PXE, as well as in the inherited form, the mineralization and fragmentation of dermal elastic fibers seem to result from the altered metabolism of mesenchymal cells, such as fibroblasts and smooth muscle cells [33, 34]. In particular, mineralization of elastic fibers occurs in the core; as the disease progresses, the outer rim becomes increasingly dense and when maximum calcification is reached fragmentation occurs [2, 35].
In the mid-dermis these abnormal and swollen fibers cause the development of yellowish papules, which can coalesce to form plaques. The lesions are predominantly located on the neck, axillae, popliteal fossae, antecubital, inguinal and periumbilical areas [36].
Ocular signs are a mottled hyperpigmentation of the retina (peau d'orange appearance) and angioid streaks, resulting from degeneration of Bruch's membrane, the elastic-rich layer of the retina [7, 29, 37, 38]. The damage of chorioid and retinal vessels can be complicated by aberrant neovascularization and retinal hemorrhages, which lead to visual impairment [38].
Vascular manifestations are caused by degeneration of the elastic laminae of medium- sized arteries. This process and the subsequent calcium deposition are associated with the early onset of peripheral vascular occlusive disease.
The origin of PXE in thalassemia and other hemolytic diseases could be attributed to an injury to the elastic tissue resulting from an oxidative process [20, 28]. In fact, in these conditions, free iron and plasma microparticles, derived from denatured hemoglobin products or oxidative damage of red blood cell membranes, are plentiful [20, 39, 40, 41]. In thalassemia iron overload is common, as a consequence of blood transfusion or of increased iron absorption. As iron loading progresses, the capacity for the transport and storage of iron may be exceeded and a non-transferrin or ferritin-bound fraction of iron may promote the generation of free radicals, which are propagators of oxidation-related damage to various organs and tissues, including elastin-rich tissues [20, 42]. The body maintains a number of antioxidant mechanisms but they may not be sufficient to prevent significant oxidative damage over a prolonged period of time in iron-overloaded patients [32].
Samarkos et al hypothesized a role for neutrophil elastase in elastic fiber degradation as suggested by elevated serum levels of elastase in in thalassemia patients, but this hypothesis has not been confirmed [31, 43].
Diffuse calcium deposition within the vascular wall is a secondary process that follows the diffuse degeneration of the elastic fibers [44]. In inherited PXE mineralized elastic fibers contain proteoglycans and glycoproteins known for their affinity for calcium ions and their involvement in bone mineralization [27, 33]. These observations, coupled with the findings that high calcium intake in early life may correlate with PXE severity [45], made Baccarani-Contri et al. to postulate calcium involvement in the mineralization process [27]. The authors also suggest that calcium precipitation in PXE could be favored by molecules preferentially associated with elastin and functioning as nucleation centers or ion-binding substrates. The sequenced region containing the PXE locus on chromosome 16 includes, among others, the genes MRP1 and MRP6 that belong to the multidrug resistance protein family, involved in extrusion of exogenous and endogenous molecules from the cells [27, 46, 47]. This transport system has been shown to be involved in cell extrusion of conjugated bilirubin, a molecule whose metabolism may be affected in beta-thalassemia [48, 49].
Finally, it has been hypothesized that the skin, ocular and vascular findings represent the evolving changes of an underlying elastic tissue disorder that starts early in life. It is known that subclinical histopathological disorders of the elastic tissue are present in the absence of clinically evident lesions from childhood. In fact, elastic fibre defects of the arterial wall were noticed in 96 percent of spleen and liver biopsies taken from thalassemia children and adolescents [19, 50, 51].
Complications
Degeneration of the elastic laminae of medium-sized arteries may lead to serious cardiovascular complications. In the inherited form, intracanial haemorrhages, ischemic strokes, coronary arterial calcifications, myocardial infarction, valvular calcification and leaflet thickening, pericardial thickening, renal artery calcification with arterial hypertension, and peripheral arterial abnormalities complicated by gastric hemorrhage and intestinal infarcts, have all been reported [7, 28, 30, 50, 52, 53, 54].
In children and teenagers bleeding complications, especially gastrointestinal hemorrhages, probably due to fragility of calcified submucosal vessels, have been described [2, 55, 56].
In the thalassemic population several cases of cardiac involvement have been reported, including rupture of chordae tendinae and aneurysmatic dilatation of the ascending aorta [28, 50, 52, 54, 57].
A case of severe aortic calcification, which necessitated valve replacement has been recently reported [44]. Because valvular calcification usually represents an aging process, the fact that it was encountered in a 46-year-old female implied the presence of an additional factor, such as the coexistent PXE-like syndrome, which accelerated the calcification process.
In the follow-up report mentioned above [30], out of 14 Italian thalassemia patients with a PXE-like syndrome, five died because of cardiovascular complications. Two deaths were caused by infarction while fatal vascular diseases were seen in three cases, all occurring from 8 to 132 months after the first observation.
Conclusions
Pseudoxanthoma Elasticum in thalassemia is an acquired and age-dependent condition with the same clinical manifestations of the genetic form, variable severity, and a generally late onset, usually after the second decade of life. The pathogenesis of PXE-like lesions in beta-thalassemia is not yet completely understood. Because several complications could be serious and life threatening, close surveillance of these patients is mandatory.
References
1. Moreira AP, Feijó FS, Pinto JM, Martinelli IL, Rochael MC. Pseudoxanthoma Elasticum. Dermatol Online J. 2009 Jan 15;15(1):7. [PubMed]2. Laube S, Moss C. Pseudoxanthoma elasticum. Arch Dis Child. 2005 Jul;90(7):754-6. [PubMed]
3. Van Soest S, Swart J, Tijmes N, Sandkuijl LA, Rommers J, Bergen AA. A locus for autosomal recessive pseudoxanthoma elasticum, with penetrance of vascular symptoms in carriers, maps to chromosome 16p13.1. Genome Res. 1997 Aug;7(8):830-4.[PubMed]
4. Le Saux O, Urban Z, Göring HH, Csiszar K, Pope FM, Richards A, Pasquali-Ronchetti I, Terry S, Bercovitch L, Lebwohl MG, Breuning M, van den Berg P, Kornet L, Doggett N, Ott J, de Jong PT, Bergen AA, Boyd CD. Pseudoxanthoma elasticum maps to an 820-kb region of the p13.1 region of chromosome 16.Genomics.1999 Nov 15;62(1):1-10. [PubMed]
5. Bergen AA, Plomp AS, Schuurman EJ, Terry S, Breuning M, Dauwerse H, Swart J, Kool M, van Soest S, Baas F, ten Brink JB, de Jong PT. Mutations in ABCC6 cause pseudoxanthoma elasticum. Nat Genet. 2000 Jun;25(2):228-31. [PubMed]
6. Ringpfeil F, Lebwohl MG, Christiano AM, Uitto J. Pseudoxanthoma elasticum: mutations in the MRP6 gene encoding a transmembrane ATP-binding cassette (ABC) transporter. Proc Natl Acad Sci U S A. 2000 May 23;97(11):6001-6. [PubMed]
7. Chassaing N, Martin L, Calvas P, Le Bert M, Hovnanian A. Pseudoxanthoma elasticum: a clinical, pathophysiological and genetic update including 11 novel ABCC6 mutations. J Med Genet. 2005 Dec;42(12):881-92. Epub 2005 May 13. [PubMed]
8. Paton D. Angiod streaks and sickle cell anemia: a report of two cases. Arch Ophthalmol. 1959 Nov;62:852-8. [PubMed]
9. Nagpal KC, Asdourian G, Goldbaum M, Apple D, Goldberg MF. Angioid streaks and sickle haemoglobinopathies. Br J Ophthalmol. 1976 Jan;60(1):31-4. [PubMed]
10. Goldberg MF, Charache S, Acacio I. Ophthalmologic manifestations of sickle cell thalassemia. Arch Intern Med. 1971 Jul;128(1):33-9. [PubMed]
11. Aessopos A, Stamatelos G, Savvides P, Kavouklis E, Gabriel L, Rombos I, Karagiorga M, Kaklamanis P. Angioid streaks in homozygous beta thalassemia. Am J Ophthalmol. 1989 Oct 15;108(4):356-9. [PubMed]
12. Gibson JM, Chaudhuri PR, Rosenthal AR. Angioid streaks in a case of beta thalassaemia major. Br J Ophthalmol. 1983 Jan;67(1):29-31. [PubMed]
13. Kinsella FP, Mooney DJ. Angioid streaks in beta thalassaemia minor. Br J Ophthalmol. 1988 Apr;72(4):303-4. [PubMed]
14. O'Donnell BF, Powell FC, O'Loughlin S, Acheson RW. Angioid streaks in beta thalassaemia minor. Br J Ophthalmol. 1991 Oct;75(10):639 [PubMed]
15. McLane NJ, Grizzard WS, Kousseff BG, Hartmann RC, Sever RJ. Angioid streaks associated with hereditary spherocytosis. Am J Ophthalmol. 1984 Apr;97(4):444-9. [PubMed]
16. Sandström H, Wahlin A, Eriksson M, Holmgren G, Lind L, Sandgren O. Angioid streaks are part of a familial syndrome of dyserythropoietic anaemia (CDA III). Br J Haematol. 1997 Sep;98(4):845-9. [PubMed]
17. Daneshmend TK. Ocular findings in a case of haemoglobin H disease. Br J Ophthalmol. 1979 Dec;63(12):842-4. [PubMed]
18. McBrayer GM, Semes L, Stephens GG. Angioid streaks and AC hemoglobinopathy--a newly discovered association. J Am Optom Assoc. 1993 Apr;64(4):250-3. [PubMed]
19. Tsomi K, Karagiorga-Lagana M, Karabatsos F, Fragodimitri C, van Vliet-Konstantinidou C, Premetis E, Stamoulakatou A. Arterial elastorrhexis in beta-thalassaemia intermedia, sickle cell thalassaemia and hereditary spherocytosis.Eur J Haematol. 2001 Sep;67(3):135-41 [PubMed]
20. Aessopos A, Farmakis D, Loukopoulos D. Elastic tissue abnormalities in inherited haemolytic syndromes. Eur J Clin Invest. 2002 Sep;32(9):640-2. [PubMed]
21. Borgna-Pignatti C, Rugolotto S, De Stefano P, Zhao H, Cappellini MD, Del Vecchio GC, Romeo MA, Forni GL, Gamberini MR, Ghilardi R, Piga A, Cnaan A. Survival and complications in patients with thalassemia major treated with transfusion and deferoxamine.Haematologica. 2004 Oct;89(10):1187-93. [PubMed]
22. Suerig KC, Siefert FE. Pseudoxanthoma elasticum and sickle cell anemia. Arch Intern Med 1964; 113: 135-41. [PubMed]
23. Aesopos A, Stamatelos G, Savvides P, Rombos I, Tassiopoulos T, Kaklamanis P. Pseudoxanthoma elasticum and angioid streaks in two cases of beta-thalassaemia. Clin Rheumatol. 1989 Dec;8(4):522-7. [PubMed]
24. Aessopos A, Savvides P, Stamatelos G, Rombos I, Tassiopoulos T, Karagiorga M, Kaklamanis P, Fessas P. Pseudoxanthoma elasticum-like skin lesions and angioid streaks in beta-thalassemia. Am J Hematol. 1992 Nov;41(3):159-64. [PubMed]
25. Aessopos A, Voskaridou E, Kavouklis E, Vassilopoulos G, Rombos Y, Gavriel L, Loukopoulos D. Angioid streaks in sickle-thalassemia. Am J Ophthalmol. 1994 May 15;117(5):589-92. [PubMed]
26. Cianciulli P, Sorrentino F, Forte L, Papa G. Pseudoxanthoma elasticum in pazienti talassemici. XXXIII Congr Naz Ematol, Napoli 1995, p.73.
27. Baccarani-Contri M, Bacchelli B, Boraldi F, Quaglino D, Taparelli F, Carnevali E, Francomano MA, Seidenari S, Bettoli V, De Sanctis V, Pasquali-Ronchetti I. Characterization of pseudoxanthoma elasticum-like lesions in the skin of patients with beta-thalassemia. J Am Acad Dermatol. 2001 Jan;44(1):33-9. [PubMed]
28. Aessopos A, Samarkos M, Voskaridou E, Papaioannou D, Tsironi M, Kavouklis E, Vaiopoulos G, Stamatelos G, Loukopoulos D. Arterial calcifications in beta-thalassemia. Angiology. 1998 Feb;49(2):137-43. [PubMed]
29. Hu X, Plomp AS, van Soest S, Wijnholds J, de Jong PT, Bergen AA. Pseudoxanthoma elasticum: a clinical, histopathological, and molecular update. Surv Ophthalmol. 2003 Jul-Aug;48(4):424-38. [PubMed]
30. Cianciulli P, Sorrentino F, Maffei L, Amadori S, Cappabianca MP, Foglietta E, Carnevali E, Pasquali-Ronchetti I. Cardiovascular involvement in thalassaemic patients with pseudoxanthoma elasticum-like skin lesions: a long-term follow-up study. Eur J Clin Invest. 2002 Sep;32(9):700-6. [PubMed]
31. Samarkos M, Aessopos A, Fragodimitri C, Karagiorga M, Kalotychou V, Voskaridou E, Kavouklis E, Loukopoulos D. Neutrophil elastase in patients with homozygous beta-thalassemia and pseudoxanthoma elasticum-like syndrome. Am J Hematol. 2000 Feb;63(2):63-7. [PubMed]
32. Hamlin N, Beck K, Bacchelli B, Cianciulli P, Pasquali-Ronchetti I, Le Saux O. Acquired Pseudoxanthoma elasticum-like syndrome in beta-thalassaemia patients. Br J Haematol. 2003 Sep;122(5):852-4. [PubMed]
33. Contri MB, Boraldi F, Taparelli F, De Paepe A, Ronchetti IP. Matrix proteins with high affinity for calcium ions are associated with mineralization within the elastic fibers of pseudoxanthoma elasticum dermis. Am J Pathol. 1996 Feb;148(2):569-77. [PubMed]
34. Baccarani-Contri M, Vincenzi D, Cicchetti F, Mori G, Pasquali-Ronchetti I. Immunochemical identification of abnormal constituents in the dermis of pseudoxanthoma elasticum patients. Eur J Histochem. 1994;38(2):111-23. [PubMed]
35. Hacker SM, Ramos-Caro FA, Beers BB, Flowers FP. Juvenile pseudoxanthoma elasticum: recognition and management. Pediatr Dermatol. 1993 Mar;10(1):19-25. [PubMed]
36. Ballabio E, Bersano A, Bresolin N, Candelise L. Monogenic vessel diseases related to ischemic stroke: a clinical approach. J Cereb Blood Flow Metab. 2007 Oct;27(10):1649-62. Epub 2007 Jun 20. [PubMed]
37. Neldner KH. Pseudoxanthoma elasticum. Int J Dermatol. 1988 Mar;27(2):98-100. [PubMed]
38. Aessopos A, Floudas CS, Kati M, Tsironi M, Giakoumi X, Livir-Rallatos C, Farmakis D. Loss of vision associated with angioid streaks in beta-thalassemia intermedia. Int J Hematol. 2008 Jan;87(1):35-8. Epub 2007 Dec 11. [PubMed]
39. Belcher JD, Marker PH, Weber JP, Hebbel RP, Vercellotti GM. Activated monocytes in sickle cell disease: potential role in the activation of vascular endothelium and vaso-occlusion. Blood. 2000 Oct 1;96(7):2451-9. [PubMed]
40. Gutteridge JM, Smith A. Antioxidant protection by haemopexin of haem-stimulated lipid peroxidation. Biochem J. 1988 Dec 15;256(3):861-5. [PubMed]
41. Hanley ME, Repine JE. Elastase and oxygen radicals: synergistic interactions. Agents Actions Suppl. 1993;42:39-47. [PubMed]
42. Hershko C, Link G, Cabantchik I. Pathophysiology of iron overload. Ann N Y Acad Sci. 1998 Jun 30;850:191-201. [PubMed]
43. Schwartz E, Benz EJJ, Forget BG. Thalassemia Syndromes. In: Hoffman R,Benz EJJ, Shattil SJ, Furie B, Cohen HJ, Silberstein LE, editors. Hematology Basic Principles and practice. 2nd edition. New York: Churchill Livingston 1995. p 586-610.
44. Farmakis D, Polonifi A, Deftereos S, Tsironi M, Papaioannou I, Aessopos A. Aortic valve replacement in a patient with thalassemia intermedia. Ann Thorac Surg. 2006 Feb;81(2):737-9. [PubMed]
45. Renie WA, Pyeritz RE, Combs J, Fine SL. Pseudoxanthoma elasticum: high calcium intake in early life correlates with severity. Am J Med Genet. 1984 Oct;19(2):235-44. [PubMed]
46. Zaman GJ, Flens MJ, van Leusden MR, de Haas M, Mülder HS, Lankelma J, Pinedo HM, Scheper RJ, Baas F, Broxterman HJ, et al. The human multidrug resistance-associated protein MRP is a plasma membrane drug-efflux pump. Proc Natl Acad Sci U S A. 1994 Sep 13;91(19):8822-6. [PubMed]
47. Kool M, van der Linden M, de Haas M, Baas F, Borst P. Expression of human MRP6, a homologue of the multidrug resistance protein gene MRP1, in tissues and cancer cells. Cancer Res. 1999 Jan 1;59(1):175-82. [PubMed]
48. Yang B, Kirby S, Lewis J, Detloff PJ, Maeda N, Smithies O. A mouse model for beta 0-thalassemia. Proc Natl Acad Sci U S A. 1995 Dec 5;92(25):11608-12. [PubMed]
49. Livrea MA, Tesoriere L, Pintaudi AM, Calabrese A, Maggio A, Freisleben HJ, D'Arpa D, D'Anna R, Bongiorno A. Oxidative stress and antioxidant status in beta-thalassemia major: iron overload and depletion of lipid-soluble antioxidants. Blood. 1996 Nov 1;88(9):3608-14. [PubMed]
50. Farmakis D, Deftereos S, Giakoumis A, Polymeropoulos E, Aessopos A. Rupture of chordae tendineae in patients with beta-thalassemia. Eur J Haematol. 2004 Apr;72(4):296-8. [PubMed]
51. Tsomi K, Karagiorga-Lagana M, Fragodimitri C, Karabatsos F, Katsiki V. Arterial elastorrhexis: manifestation of a generalized elastic tissue disorder in beta-thalassaemia major. Eur J Haematol. 1999 Nov;63(5):287-94. [PubMed]
52. Aessopos A, Farmakis D, Karagiorga M, Voskaridou E, Loutradi A, Hatziliami A, Joussef J, Rombos J, Loukopoulos D. Cardiac involvement in thalassemia intermedia: a multicenter study. Blood. 2001 Jun 1;97(11):3411-6. [PubMed]
53. Aessopos A, Farmakis D, Karagiorga M, Rombos I, Loucopoulos D. Pseudoxanthoma elasticum lesions and cardiac complications as contributing factors for strokes in beta-thalassemia patients. Stroke. 1997 Dec;28(12):2421-4. [PubMed]
54. Farmakis D, Moyssakis I, Perakis A, Rombos Y, Deftereos S, Giakoumis A, Polymeropoulos E, Aessopos A. Unstable angina associated with coronary arterial calcification in a thalassemia intermedia patient with a pseudoxanthoma elasticum-like syndrome. Eur J Haematol. 2003 Jan;70(1):64-6. [PubMed]
55. Schachner L, Young D. Pseudoxanthoma elasticum with severe cardiovascular disease in a child. Am J Dis Child. 1974 Apr;127(4):571-5. [PubMed]
56. Heaton JP, Wilson JW. Pseudoxanthoma elasticum and its urological implications. J Urol. 1986 Apr;135(4):776-7. [PubMed]
57. Farmakis D, Vesleme V, Papadogianni A, Tsaftaridis P, Kapralos P, Aessopos A. Aneurysmatic dilatation of ascending aorta in a patient with beta-thalassemia and a pseudoxanthoma elasticum-like syndrome. Ann Hematol. 2004 Sep;83(9):596-9. Epub 2004 Mar 11. [PubMed]
© 2009 Dermatology Online Journal

