Treatment of gingival fibromas using CO laser and electrosurgery in a patient with tuberous sclerosis
Published Web Location
https://doi.org/10.5070/D318t4d86nMain Content
Treatment of gingival fibromas using CO2 laser and electrosurgery in a patient with tuberous sclerosis
Daniel B Eisen MD1, Nasim Fazel MD DDS2
Dermatology Online Journal 14 (11): 7
1. Assistant Clinical Professor of Dermatology, Department of Dermatology, University of California Davis Medical Center,
Sacramento, CA. dbeisen@ucdavis.edu2. Assistant Clinical Professor of Dermatology, Department of Dermatology, University of California Davis Medical Center, Sacramento, CA
Abstract
A 10-year-old boy with a history of tuberous sclerosis was sent for evaluation of numerous papules on his lower gum area. The parent was concerned that the lesions were interfering with oral hygiene. A diagnosis of oral fibromas was made and treatment options of gingivectomy or electrosurgery combined with carbon dioxide laser were described to the patient and his parent. Therapy with electrocautery and a pulsed carbon dioxide laser was decided on and utilized. We describe for the first time the combination of electrosurgery and carbon dioxide laser as a treatment method for oral fibromas. A short review of the literature regarding diagnosis and treatment is included with this report.
Introduction
Oral angiofibromas are a minor criteria for the diagnosis of tuberous sclerosis. Traditional treatment for these lesions has been gingevectomy. Treatment options for these lesions have not been well studied. We report a patient treated with a combination of electrosurgery and carbon dioxide laser.
Report of Case
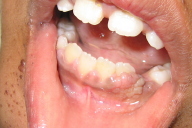
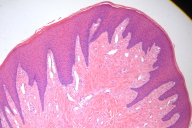
A 10-year-old boy was sent for evaluation of numerous papules on his lower gum area (Fig. 1). A shave biopsy from the mandibular gingiva, which was performed prior to consultation, confirmed the lesions to be angiofibromas (Fig. 2). The parent was concerned that the lesions were interfering with oral hygiene. Treatment options of gingivectomy and electrosurgery combined with carbon dioxide laser were described to the patient and his parent. The parent decided on treatment with electrosurgery and carbon dioxide laser. After obtaining anesthesia using intraoral mandibular nerve blocks, the biggest lesions were debulked using electrocautery. The debris was then wiped away using sterile gauze pads. The carbon dioxide laser was then used to ablate the residual remaining lesions with the following treatment parameters: 2-3 passes, 18 percent overlapping pulses, 250 mj/sec, 31.2 W, shape #3, size 1. The patient tolerated the procedure well and was completely healed nine days following therapy (Fig. 3). There was no evidence of gum recession or damage to the teeth enamel. The patient was seen in follow-up 24 months following the procedure with only minimal recurrence of the lesions (Fig. 4).
 |  |
| Figure 3 | Figure 4 |
|---|---|
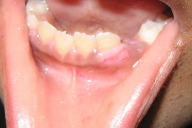
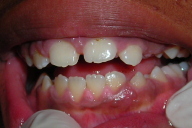
| Figure 3. Appearance of gingiva 9 days after a single session of electrosurgery and carbon dioxide laser ablation. Figure 4. Appearance of gingiva 24 months after the procedure. | |
Discussion
Tuberous sclerosis (Bourneville disease) is an autosomal dominant disease characterized by the triad of epilepsy, low intelligence and facial angiofibromas (adenoma sebaceum) [1]. However, the full triad of symptoms is present in only a minority of patients. Tuberous sclerosis has an estimated prevalence of 1:5800 [2]. Major features of the disease include: facial angiofibromas, ungual or periungual fibromas, hypomelanotic macules, shagreen patch (connective tissue nevus), multiple retinal nodular hamartomas, cortical tubers, subependymal nodules, subependymal giant cell astrocytomas, cardiac rhabdomyomas, lymphangioleiomyomatosis, renal angiomyolipomas [3]. Minor criteria for the disease include dental enamel pits, hamartomas, rectal polyps, bone cysts, cerebral white matter, radial migration lines, gingival fibromas, nonrenal hamaroma, retinal achromic patch, confetti ski lesions, multiple renal cysts.
Gingival fibromas associated with tuberous sclerosis have been documented in only a few case reports, but they are considered a minor criterion for the diagnosis of the disorder. Their prevalence in patients with tuberous sclerosis is unknown. The differential diagnosis of these papules includes: gingival fibrous nodule, papilloma, focal epithelial hyperplasia, fibroma, gingival cyst, multiple hamartomas, and exostosis[4].
Treatment regarding oral angiofibromas has not been well studied [5]. However, there are many descriptions in the literature detailing the use of CO2 laser for facial and periungual fibromas [6-13]. The CO2 laser is used in oral medicine most often in the setting of phenytoin, or cyclosporine induced gingival overgrowth [14-17]. The laser has the following advantages in the setting of oral surgery: hemostasis, reduced postoperative swelling, reduced bacterial concentration at the surgery site, reduced need for suturing, quicker healing, and less post operative pain [14, 17-27].
Our case adds to the sparse literature on treatments for oral angiofibromas. The use of electrosurgery and carbon dioxide laser ablation is ease to perform and appears to be effective and well tolerated. It should be considered as a treatment option for patients with this disorder.
References
1. Roach, E.S., M.R. Gomez, and H. Northrup, Tuberous sclerosis complex consensus conference: revised clinical diagnostic criteria. J Child Neurol, 1998. 13(12): p. 624-8. PubMed2. Webb, D.W., A.E. Fryer, and J.P. Osborne, On the incidence of fits and mental retardation in tuberous sclerosis. J Med Genet, 1991. 28(6): p. 395-7. PubMed
3. Hyman, M.H. and V.H. Whittemore, National Institutes of Health consensus conference: tuberous sclerosis complex. Arch Neurol, 2000. 57(5): p. 662-5. PubMed
4. Giunta, J.L., Gingival fibrous nodule. Oral Surg Oral Med Oral Pathol Oral Radiol Endod, 1999. 88(4): p. 451-4. PubMed
5. Spenler, C.W., B.M. Achauer, and V.M. Vander Kam, Treatment of extensive adenoma sebaceum with a carbon dioxide laser. Ann Plast Surg, 1988. 20(6): p. 586-9. PubMed
6. Berlin, A.L. and R.C. Billick, Use of CO2 laser in the treatment of periungual fibromas associated with tuberous sclerosis. Dermatol Surg, 2002. 28(5): p. 434-6. PubMed
7. Song, M.G., K.B. Park, and E.S. Lee, Resurfacing of facial angiofibromas in tuberous sclerosis patients using CO2 laser with flashscanner. Dermatol Surg, 1999. 25(12): p. 970-3. PubMed
8. Weston, J., et al., Carbon dioxide laserbrasion for treatment of adenoma sebaceum in tuberous sclerosis. Ann Plast Surg, 1985. 15(2): p. 132-7. PubMed
9. Boixeda, P., et al., CO2, argon, and pulsed dye laser treatment of angiofibromas. J Dermatol Surg Oncol, 1994. 20(12): p. 808-12. PubMed
10. Bellack, G.S. and S.M. Shapshay, Management of facial angiofibromas in tuberous sclerosis: use of the carbon dioxide laser. Otolaryngol Head Neck Surg, 1986. 94(1): p. 37-40. PubMed
11. Janniger, C.K. and D.J. Goldberg, Angiofibromas in tuberous sclerosis: comparison of treatment by carbon dioxide and argon laser. J Dermatol Surg Oncol, 1990. 16(4): p. 317-20. PubMed
12. Papadavid, E., et al., Carbon dioxide and pulsed dye laser treatment of angiofibromas in 29 patients with tuberous sclerosis. Br J Dermatol, 2002. 147(2): p. 337-42. PubMed
13. Bittencourt, R.C., et al., Treatment of angiofibromas with a scanning carbon dioxide laser: a clinicopathologic study with long-term follow-up. J Am Acad Dermatol, 2001. 45(5): p. 731-5. PubMed
14. Pick, R.M., B.C. Pecaro, and C.J. Silberman, The laser gingivectomy. The use of the CO2 laser for the removal of phenytoin hyperplasia. J Periodontol, 1985. 56(8): p. 492-6. PubMed
15. Hylton, R.P., Use of CO2 laser for gingivectomy in a patient with Sturge-Weber disease complicated by dilantin hyperplasia. J Oral Maxillofac Surg, 1986. 44(8): p. 646-8. PubMed
16. Guelmann, M., L.R. Britto, and J. Katz, Cyclosporin-induced gingival overgrowth in a child treated with CO2 laser surgery: a case report. J Clin Pediatr Dent, 2003. 27(2): p. 123-6. PubMed
17. Roed-Petersen, B., The potential use of CO2-laser gingivectomy for phenytoin-induced gingival hyperplasia in mentally retarded patients. J Clin Periodontol, 1993. 20(10): p. 729-31. PubMed
18. Coleton, S., Lasers in surgical periodontics and oral medicine. Dent Clin North Am, 2004. 48(4): p. 937-62, vii. PubMed
19. Convissar, R.A. and E.E. Goldstein, An overview of lasers in dentistry. Gen Dent, 2003. 51(5): p. 436-40. PubMed
20. Schuller, D.E., Use of the laser in the oral cavity. Otolaryngol Clin North Am, 1990. 23(1): p. 31-42. PubMed
21. Rossmann, J.A., et al., Effects of CO2 laser irradiation on gingiva. J Periodontol, 1987. 58(6): p. 423-5. PubMed
22. Barak, S. and I. Kaplan, The CO2 laser in the excision of gingival hyperplasia caused by nifedipine. J Clin Periodontol, 1988. 15(10): p. 633-5. PubMed
23. Pecaro, B.C. and W.J. Garehime, The CO2 laser in oral and maxillofacial surgery. J Oral Maxillofac Surg, 1983. 41(11): p. 725-8. PubMed
24. Apfelberg, D.B., et al., Benefits of the CO2 laser in oral hemangioma excision. Plast Reconstr Surg, 1985. 75(1): p. 46-50. PubMed
25. Pogrel, M.A., The carbon dioxide laser in soft tissue preprosthetic surgery. J Prosthet Dent, 1989. 61(2): p. 203-8. PubMed
26. Moritz, A., et al., Treatment of periodontal pockets with a diode laser. Lasers Surg Med, 1998. 22(5): p. 302-11. PubMed
27. Abt, E., CO2 laser treatment for gingivectomies reduces hemorrhaging, post-op pain. Clin Laser Mon, 1992. 10(1): p. 8. PubMed
© 2008 Dermatology Online Journal