Multinucleate cell angiohistiocytoma: Report of three new cases and literature review
Published Web Location
https://doi.org/10.5070/D30z27t0xzMain Content
Multinucleate cell angiohistiocytoma: Report of three new cases and literature review
Laure Jaconelli, Jean Kanitakis, Salma Ktiouet, Michel Faure, Alain Claudy
Dermatology Online Journal 15 (2): 4
Dept. of Dermatology, Ed. Herriot Hospital, 69437 Lyon cx 03, France. jean.kanitakis@univ-lyon1.frAbstract
Multinucleate cell angiohistiocytoma (MCA) is a rare benign vascular proliferation of the skin of unknown cause. About 75 cases have been reported previously. We present herein three new cases of MCA studied immunohistologically and present a review the relevant literature that delineates the salient clinicopathological features of this unusual entity.
Introduction
Multinucleate cell angiohistiocytoma (MCA) is a rare benign proliferation of the skin of unknown etiology and it shows some degree of clinical and histological heterogeneity. It was originally described in 1985 [1, 2] and about 75 cases have been reported in the literature. We present herein three new cases of MCA studied immunohistologically and review the salient clinicopathological features.
Case reports
Patient 1
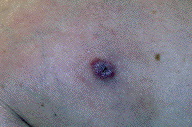
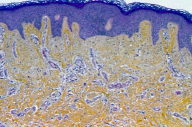
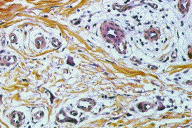
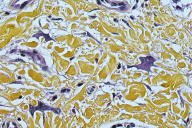
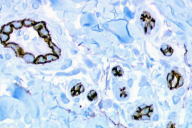
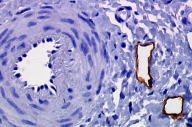
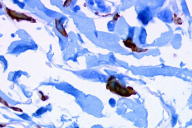
A 55-year-old woman presented with an asymptomatic firm, dome-shaped nodule of the lower third of the inner right thigh (Fig. 1). The lesion measured 1 cm in diameter, was pink-red in color, and had been present for several months. The patient was otherwise healthy and reported no significant skin problems. The nodule was surgically excised under local anesthesia; the presumed clinical diagnosis was angioma or dermatofibroma. Microscopic examination showed a proliferative dermal lesion below a moderately thickened epidermis (Fig. 2). The upper and mid dermis contained a diffuse proliferation of small-sized vessels with thick walls. Each vessel had a round or elongated lumen lined by endothelial cells that were often plump (Fig. 3). The surrounding dermis was composed of slightly homogenized collagen bundles and contained a moderately dense cell infiltrate of lymphohistiocytic cells forming perivascular cuffs. Several large multinucleated cells with bizarre, mostly angulated contours were interspersed between collagen bundles (Fig. 4).
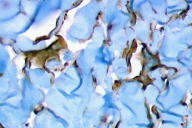
Perls stain was negative. Immunohistochemically, endothelial cells of the proliferative vessels expressed the endothelial antigens CD31, CD34 (Fig. 5), thrombomodulin and BNH9, and more weakly and focally HLA-DR antigens, but not podoplanin (D2-40), expressed by lymphatic endothelial cells (Fig. 6). The multinucleate cells expressed vimentin (Fig. 7) and, more variably, antigens of the monocyte/macrophage lineage such as CD68, calprotectin/Mac 387, and CD163 (Fig. 8); they did not express endothelial antigens, Factor XIIIa, S100 protein, CD1a, CD43, CD45, CD79, CD117, Ki67, smooth muscle actin, bcl2 oncoprotein, or the LAN antigen of HHV8.
Patient 2
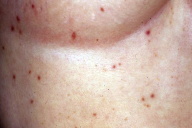 |
| Figure 9 |
|---|
| Figure 9. Red papular lesions scattered over the chest (Patient 2). |
A 50 year-old-woman presented with a 3-year history of several rapidly growing red-brown papules disseminated on the chest and both arms (Fig. 9) that continued to spread despite treatment with topical corticosteroids. Her medical history included vitiligo on the face and dorsum of the hands. Laboratory work-up disclosed anti-thyroid antibodies. Histological examination of a lesion of the trunk showed a moderately acanthotic epidermis. The mid-dermis contained a proliferation of small to medium-sized vessels with plump endothelial cells, surrounded by a moderately dense lymphoid infiltrate with occasional plasma cells. The adjacent dermis contained numerous bizarre multinucleated cells with scalloped margins. Immunohistochemically, CD31 and CD34 were expressed by vascular endothelial cells. Factor XIIIa was expressed by numerous dermal interstitial cells, some of which were multinucleated and exhibited ample cytoplasm.
Patient 3
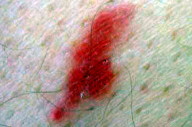 |
| Figure 10 |
|---|
| Figure 10. Red papular lesion of the thigh (Patient 3). |
A 72-year-old man with an unremarkable past medical history presented with a 3 x 3 cm asymptomatic erythematous infiltrated plaque on his left thigh that had been slowly growing over 3 years. Two similar plaques developed on the right thigh one year later (Fig. 10). The first lesion was surgically excised under local anesthesia. Histological examination showed a dermal vascular proliferation, surrounded by a rather dense lymphoid infiltrate with several giant multinucleate cells. Several dermal cells expressed the Factor XIIIa antigen and heterogeneous positivity was noted for the bizarre giant cells. Some of the later expressed the CD68 Antigen (PGM1).
Discussion
Multinucleate cell angiohistiocytoma (MCA) is an unusual, but probably under-recognized, benign vascular proliferation; about 75 cases have been reported so far. Multinucleate cell angiohistiocytoma shows a predilection for adult women (F:M ratio 3:1) and manifests as single or multiple (up to 10) firm, red-brown or violaceous papules with a smooth, or occasionally, scaly surface [3]. The lesions may have a plaque-like appearance [4]; they usually measure less than 1 cm and develop insidiously over several months or years. They appear mostly over the distal limbs, especially the dorsum of hands, wrists, thighs, and legs, but can also be seen on the face, including the forehead [5, 6], cheeks [7], orbit [8], lower lid [9], upper lip [10], and trunk [4, 11-13, our 2nd case]. Very recently, a mucosal location (oral cavity) was reported [14]. The lesions are rarely disseminated or generalized [6, 15]. When multiple, the lesions are usually grouped over the same area, showing a random, annular [6, 16] or linear [17], distribution, which may sometimes be bilateral. The lesion may be solitary [4, our 1st case]. Multinucleate cell angiohistiocytoma is as a rule asymptomatic, but may occasionally cause pruritus [13, 16, 18]. No triggering factors (such as local trauma) are usually recalled. The lesions grow slowly. Spontaneous regression was observed in at least two cases [11, 19]. Multinucleate cell angiohistiocytoma is invariably benign and appears in otherwise healthy persons. Some reported associations such as mycosis fungoides [20], diabetes mellitus [21], or vitiligo (our 2nd case) seem fortuitous.
The diagnosis of MCA is usually made by histological examination. Typically,the upper and mid dermis show a vascular proliferation predominantly of capillaries and venules. The vessel lumen may be dilated or narrowed because of the protrusion of the large nuclei of endothelial cells. The surrounding dermis consists of thickened collagen bundles and contains mononuclear cells with a fibrohistiocytic appearance. The characteristic cells are large, with irregular, bizarre shapes and scalloped or angular margins. They contain multiple, occasionally hyperchromatic, nuclei. A perivascular and perifollicular cell infiltrate is often present, made up of lymphocytes, histiocytes, and occasional neutrophils and plasma cells. The overlying epidermis is normal or hyperplastic [13, 16, 19, 22, our cases 1 & 2]. There is no hemorrhage; stains for hemosiderin (Perls) are negative. Some authors have reported a decreased elastic fiber network, shown with orcein staining [7]. Some cases exhibited an increased number of mast cells in the close vicinity of multinucleated cells and it has been hypothesized that these mast cells could play a role in the formation of the multinucleated cells [12]. Immunohistochemically, endothelial cells of the proliferative vessels express vascular endothelial antigens such as CD31, CD34, von Willebrand factor, BMA 120, EN4, and the lectin UEA-I [9, 11]. The perivascular lymphocytic infiltrate is made of CD3+/CD4+, and rarely CD8+ cells. Interstitial mononuclear cells express vimentin, Factor XIIIa, lysozyme, alpha-1-antitrypsin, and HLA-DR antigens, but not S100 protein or CD1a. Mac 387/calprotectin is expressed by a minority of cells (<10%), if at all [7, 11, 12, 22]. The characteristic multinucleated cells express vimentin but usually none of the previous markers of the monocyte/macrophage lineage [7, 9, 11, 16, 22]. The expression of CD68 was found to be variably positive in some cases [6, 12, 23, our cases 1 and 3] and negative in others [15, 22]. Direct immunofluorescence, performed in one case [7], showed microgranular deposits of IgE at the dermal-epidermal junction, but the significance of this finding is unclear.
The clinical appearance of MCA is not really specific since several entities can have a similar appearance. The most common of them include:
- Kaposi sarcoma (KS): Microscopically, KS consists of irregularly-anastomosing vascular channels and slits with a sieve-like appearance, hemorrhage and hemosiderin deposits, and spindle-shaped cells. Occasionally, the promontory sign is observed but multinucleated cells are not a feature. Kaposi sarcoma cells express podoplanin, a marker of lymphatic endothelium [24], which, according to our results, is not expressed by endothelial cells of MCA. The presence of HHV8 (shown with immunohistochemistry or in situ hybridization) readily allows the distinction of KS from MCA. The two entities have different properties in vitro. As opposed to KS cells, MCA cells have a reduced life span, are not able to cross basement membranes, cannot be amplified, and do not harbor HHV8 [22].
- Acroangiodermatitis (pseudo-KS): This affects patients with venous insufficiency who usually have venous stasis. Clinically this usually presents as purpuric papules and plaques over the distal lower legs and feet. Microscopically, pseudo-KS shows tortuous dermal capillaries with thick walls, oriented perpendicular to the skin surface, and heavy hemosiderin deposits within surrounding macrophages.
- Granuloma annulare, lichen planus, lobular hemangioma, angio-lymphoid hyperplasia with eosinophilia, bacillary angiomatosis, sarcoidosis, lipoid necrobiosis, lupus erythematosus, erythema elevatum diutinum, and lymphocytic infiltration of the skin may occasionally mimic MCA. Differentiation requires microscopic examination.
Histologically the differential diagnosis of MCA may be subtle and more difficult to distinguish from the following entities:
- Varieties of angiofibromas: Fibrous papule of the nose usually presents as a single papule of the face (nose). In angiofibromas collagen bundles are oriented vertically and often form onion-skin-like concentric lamellae around hair follicles and blood vessels. The dermis contains abundant dilated capillaries lined by an inconspicuous endothelial layer but only occasionally multinucleated cells.
- Dermatofibromas: These are characterized by more prominent epidermal hyperplasia and hyperpigmentation with a higher density of proliferating dermal cells. The vascular atrophic variety of dermatofibroma contains occasionally multinucleated cells and prominent vessels, but the dermis is thinned and contains a denser histiocytic cell infiltrate.
- Microvenular hemangioma is typically located on the legs of young adults and consists of a proliferation of irregularly-shaped, branching venules within hyalinized collagen bundles. These vessels are lined by endothelial cells with prominent nuclei and are surrounded by flattened pericytes. Giant cells are absent.
The precise origin of the multinucleated cells in MCA is not known with certainty. Some authors have suggested a fibroblastic origin for these cells based on their ultrastructural appearance (presence of thickenings of the nuclear membrane "zonula nucleus limitans," prominent endoplasmic reticulum) [9, 12] and by the usual (although equivocal) lack of monocyte/macrophage markers [13, 22]. However, the occasional presence of endocytotic/pinocytotic activity and of numerous lysosomes (supported by the immunohistochemical expression of CD68 noted on several occasions) is suggestive of a histiocytic/macrophagic origin [12, 19, our cases]. The heterogeneity of CD68 expression (within the same lesion or between different patients) could be due to different maturation or degeneration stages of the multinucleated cells. We observed in our first case that multinucleated cells express CD163, a scavenger receptor expressed by tissue macrophages [25]. This finding supports the contention that MCA cells belong to the monocyte/macrophage lineage. On the other hand, it has been suggested that mast cells, found within MCA lesions, interact with mononuclear Factor XIII+ interstitial dendritic cells and play a role (through cytokine secretion and/or direct cell-cell interactions) in the vascular hyperplasia characteristic of the lesion. These mast cells may also be involved in the production of multinucleated cells, namely via IL4 secretion [12]. The view has also been expressed that multinucleated cells are degenerate or defective connective tissue macrophages that have become mitotically and functionally inactive under conditions of chronic stimulation [11]. According to some authors, Factor XIII+ dermal dendrocytes (found in normal skin around dermal blood vessels) could proliferate under the influence of inflammatory, post-traumatic, or immunological stimuli and induce the vascular proliferation found in MCA [23].
The available data suggest that MCA is a benign entity showing a slowly progressive, but indolent course. The absence of extracutaneous involvement and malignant transformation, the possibility of spontaneous regression, and the lack of histological criteria of malignancy are consistent with a reactive inflammatory process rather than a truly neoplastic one. However, the precise cause of MCA remains unknown. The usual localization of lesions over the extremities (hands, face…) suggests the role of local triggers (such as trauma or insect bites); on the other hand, the female predominance suggests a facilitating role of hormonal factors.
The treatment of MCA is not mandatory. In cases of cosmetic concern, the lesions may be excised surgically and do not appear to recur. Argon laser gives satisfactory results and is well tolerated [5]. Cryosurgery was reportedly effective in one case [18].
In conclusion, multinucleate cell angiohistiocytoma is a rarely reported entity whose clinicopathological features have been gradually delineated since its original description more than 20 years ago. This seems to be a distinct, benign, inflammatory-reactive entity belonging to the spectrum of dermal vasculoproliferative processes. Further cases need to be studied in order to define its precise origin and nature.
References
1. Smith N, Wilson Jones E. Multinucleate cell angiohistiocytoma: a new entity. Br J Dermatol. 1985;113:15.2. Smith N, Wilson Jones E. Multinucleate cell angiohistiocytoma: a new entity. J Cutan Pathol. 1986;113:15.
3. Belgodere X, Wechsler J, Pasqualini G, Paoli M. Angiohistiocytome à cellules multinucléées. Ann Dermatol Venereol. 1999;126:431-2. [PubMed]
4. Issa AA, Lui H, Shapiro J, Trotter MJ. Plaque-type multinucleate cell angiohistiocytoma. J Cutan Med Surg. 1998;3:112-4. [PubMed]
5. Kopera D, Smolle J, Kerl H. Multinucleate cell angiohistiocytoma: treatment with argon laser. Br J Dermatol. 1995;133:308-10. [PubMed]
6. Leclerc S, Clerici T, Rybojad M, Girszyn N, Morel P, Janin A, Vignon-Pennamen MD. Multinucleate cell angiohistiocytoma. Ann Dermatol Venereol. 2005;132(6-7 Pt1):546-9. [PubMed]
7. Annessi G, Girolomoni G, Giannetti A. Multinucleate cell angiohistiocytoma. Am J Dermatopathol. 1992;14:340-4. [PubMed]
8. Shields JA, Eagle RC Jr, Shields CL, Sohmer KK. Multinucleate cell angiohistiocytoma of the orbit. Am J Ophthalmol. 1995;120:402-3. [PubMed]
9. Smolle J, Auboeck L, Gogg-Retzer I, Soyer HP, Kerl H. Multinucleate cell angiohistiocytoma: a clinicopathological, immunohistochemical and ultrastructural study. Br J Dermatol. 1989;121:113-21. [PubMed]
10. Jones AC, Mullins D, Jimenez F. Multinucleate cell angiohistiocytoma of the upper lip. Oral Surg Oral Med Oral Pathol. 1994;78:743-7. [PubMed]
11. Jones WE, Cerio R, Smith NP. Multinucleate cell angiohistiocytoma: an acquired vascular anomaly to be distinguished from Kaposi's sarcoma. Br J Dermatol. 1990;122:651-63. [PubMed]
12. Puig L, Fernandez-Figueras MT, Bielsa I, Lloveras B, Alomar A. Multinucleate cell angiohistiocytoma: a fibrohistiocytic proliferation with increased mast cell numbers and vascular hyperplasia. J Cutan Pathol. 2002;29:232-7. [PubMed]
13. Väkevä L, Saksela O, Kariniemi AL. Multinucleate cell angiohistiocytoma: a report of four cases in Finland. Acta Derm Venereol. 2003;83:222-3. [PubMed]
14. Rawal YB, Anderson KM, Rawal SY. Multinucleate cell angiohistiocytoma: an uncommon mucosal tumour. Clin Exp Dermatol. 2008 Nov 24 [Epub ahead of print] [PubMed]
15. Chang SN, Kim HS, Kim SC, Yang WI. Generalized multinucleate cell angiohistiocytoma. J Am Acad Dermatol. 1996;35(2 Pt 2):320-2. [PubMed]
16. Cribier B, Gambini C, Rainero M, Grosshans E. Multinucleate cell angiohistiocytoma. A review and report of four cases. Acta Derm Venereol. 1995;75:337-9. [PubMed]
17. Aloi F, Solaroli C, Tomasini C, Pippione M. Multinucleate cell angiohistiocytoma: a report of two cases. J Eur Acad Dermatol Venereol. 1998;11:51-4. [PubMed]
18. Perez L, Zulaica A, Rodriguez L, Campo M, Penaranda J, Fernandez-Redondo V, Toribio J. Multinucleate cell angiohistiocytoma. Report of five cases. J Cutan Pathol. 2006;133:496-7. [PubMed]
19. Shapiro PE, Nova MP, Rosmarin LA, Halperin AJ. Multinucleate cell angiohistiocytoma: a distinct entity diagnosable by clinical and histologic features. J Am Acad Dermatol. 1994;30:417-22. [PubMed]
20. Bader RS, Telang GH, Vonderheid EC. Multinucleate-cell angiohistiocytoma occurring in a patient with mycosis fungoides. Cutis. 1999;63:145-8. [PubMed]
21. Zaraa I, Maubec E, Vignon-Penamen MD, Marinho E, Matichard E, Descamps V, Crickx B. Angio-histiocytome à cellules multinucléées. Ann Dermatol Venereol. 2006;133:46-7. [PubMed]
22. Sass U, Noel JC, Andre J, Simonart T. Multinucleate cell angiohistiocytoma: report of two cases with no evidence of human herpesvirus-8 infection. J Cutan Pathol. 2000;27:258-61. [PubMed]
23. Le Cam-Savin C, Dallot A, Chemaly P, Martin A, Choudat L, Amouroux J. Angiohistiocytome à cellules multinucléées. A propos de 6 cas. Ann Pathol. 1996;16:435-8. [PubMed]
24. Ordonez N. Podoplanin: a novel diagnostic immunohistochemical marker. Adv Anat Pathol. 2006;13:83-8. [PubMed]
25. Zaba L, Fuentes-Duculan J, Steinman R, Krueger J, Lowes M. Normal human dermis contains distinct populations of CD11c+BDCA1+ dendritic cells and CD163+FXIIIa+ macrophages. J Clin Invest. 2007;117:2517-25. [PubMed]
© 2009 Dermatology Online Journal