Three cases of toxic skin eruptions due to methotrexate and a compilation of methotrexate induced skin eruptions
Published Web Location
https://doi.org/10.5070/D30nq2c0bxMain Content
Three cases of toxic skin eruptions associated with methotrexate and a compilation of methotrexate-induced skin eruptions
Noah Scheinfeld
Dermatology Online Journal 12 (7): 15
Dept of Dermatology St Lukes Roosevelt Hospital Center, New York, NY. Scheinfeld@earthlink.net
Abstract
Methotrexate is an antimetabolite used as a treatment for a variety of neoplastic and inflammatory diseases. It has a variety of cutaneous side effects, in particular when administered in high doses. This is a report of cutaneous eruptions in three patients treated with methotrexate and a review of eruptions linked to methotrexate.
Methotrexate (MTX) is an antimetabolite that inhibits folic acid synthesis by blocking the function of dihydrofolic acid reductase. Dihydrofolates must be reduced to tetrahydrofolates by dihydrofolic acid reductase before they can be utilized as carriers of one-carbon groups in the synthesis of purine nucleotides and thymidylate. By inhibiting utilization of folic acid, methotrexate interferes with DNA synthesis, DNA repair, and cellular replication in rapidly proliferating cells.
Methotrexate is a chemotherapeutic mainstay. It is indicated for treatment of gestational choriocarcinoma, chorioadenoma destruens (an invasive form of hydatidiform mole), and hydatidiform mole. Methotrexate alone or in combination with other anticancer agents treats breast cancer, epidermoid cancers of the head and neck, advanced mycosis fungoides, lung cancer (squamous cell and small cell neoplasms), and squamous cell cancers including keratoacanthomas. Methotrexate is also used in combination with other chemotherapeutic agents in the treatment of advanced-stage non-Hodgkin lymphomas, Burkitt lymphoma, and lymphosarcomas [1].
Methotrexate ameliorates many inflammatory diseases. The Food and Drug Administration has given methotrexate indications for the treatment of psoriasis, rheumatoid arthritis, and juvenile rheumatoid arthritis. Methotrexate has also been used to treat pityriasis rubra pilaris, Mucha-Haberman disease, Reiter disease, pemphigus vulgaris, bullous pemphigoid, dermatomyositis, systemic lupus erythematosus, scleroderma, sarcoidosis, leukocytoclastic vasculitis, cutaneous polyarteritis nodosa, Bechet disease, pyoderma gangrenosum, atopic dermatitis, sarcoidosis, keloids, lymphomatoid papulosis, and psoriatic arthritis. It has also been used more recently as an abortifacient alone or in combination with misoprostol.
Methotrexate has many side effects. The most common side effects are liver function abnormalities, hepatitis, bone marrow suppression, nausea, gastric complaints, and hair loss [2]. Several articles published more than a decade ago and sections of dermatology textbooks elaborate the side effects of methotrexate [3, 4, 5]. This article describes three patients with cutaneous side effects to methotrexate whose underlying presentation and disease makes their case notable and reviews the cutaneous side effects of methotrexate.
Clinical synopsis
Case 1
A 46 year-old HIV-positive man had Burkitt lymphoma diagnosed 8 months previously, which involved his central nervous system. He had been treated with cyclophosphamide, doxorubicin (adriamycin/hydroxydoxorubicin), vincristine (oncovin), and prednisolone (CHOP) and methotrexate and more recently with high dose methotrexate monotherapy that resulted in decreased hoarseness and increased upper extremity strength. He presented for additional high-dose methotrexate monotherapy. The patient had been taking highly active antiretroviral therapy for months prior to presentation, which consisted of didanosine, efavirenz, tenofovir. He was given 6 grams of methotrexate intravenously over 4 hours. He also was given folic acid 1mg daily. Serum MTX was approximately 150 microM at its peak.

|
|
| Figure 1 | Figure 2 |
|---|---|
|
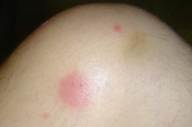
Figure 1. Linear and rippled (livedo-like) erythema was on back of Patient 1 Figure 2. Nodule on knee of Patient 1 |
|
The day after therapy ceased the patient developed a fever of 38.5° C. Blood cultures were taken and the patient was placed on vancomycin. The following day gram positive cocci in clusters grew out of one of four bottles. An eruption of linear and rippled erythema was noted on the patient's back (Fig. 1) and nodules were noted on his knees (Fig. 2). There were areas of macular erythema present on the torso clinically most consonant with a medication eruption. Biopsies were done of these eruptions and demonstrated interface dermatitis with a few necrotic keratinocytes associated with a superficial perivascular infiltrate of lymphocytes and eosinophils, focal parakeratosis on the back, and interface dermatitis with numerous necrotic keratinocytes and parakeratosis associated with a superficial perivascular infiltrate of lymphocytes and eosinophils on the knees. The patient was treated with topical betamethasone twice daily. He became afebrile and was discharged 2 days later. The eruption had resolved 2 weeks later when the patient was reexamined.
Case 2
A 25-year-old woman was 8 weeks pregnant when she decided to terminate her pregnancy. She had no medical problems. As the pregnancy was still in its early stages the patient decided to have a medical rather than a surgical abortion. She was given 50 mg per square meter of body-surface area of methotrexate intramuscularly for a total dose of 75 mg. After 2 days the patient presented with erosions on her lips (Fig. 3), bullae on her hands (Fig. 4), palmar erythema (Fig. 5), and a folliculitis on her legs (Fig. 6). She had a low grade fever (38° C) with no lymphadenopathy and was admitted to the hospital. All of her cultures were negative and no skin biopsy was performed because a self-limited toxic reaction to methotrexate was strongly suspected. Serum concentrations of methotrexate and metabolites were not done. The patient was not given misoprostol because an ultrasound demonstrated that her pregnancy had been successfully terminated. The patient eruption did not progress and she was discharged. A month later her skin examination was unremarkable.

|

|
| Figure 3 | Figure 4 |
|---|

|

|
| Figure 5 | Figure 6 |
|---|---|
|
Figure 3. Erosions on lips of Patient 2 Figure 4. Bullae on fingers of Patient 2 Figure 5. Palmar erythema of Patient 2 Figure 6. Folliculitis on thigh of Patient 2 |
|
Case 3
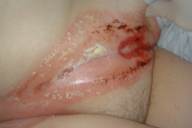
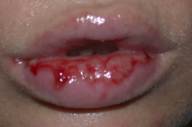
After a full-term pregnancy, a 30-year-old women had abnormal uterine bleeding. Based on elevated serum β-human chorionic gonadotrophin (β-HCG), ultrasound and biopsies of the uterus, she was diagnosed with Federation of Gynecology and Obstetrics Stage-I choriocarcinoma. A computerized tomography of the body did not reveal evidence of metastatic disease. The patient was treated with methotrexate 30 mg daily intravenously for 5 days. The patient was also given folic acid. On day 3 the patient developed erosions and hemorrhagic bullae around her vagina and abdominal fold (Fig. 7) and erosions on her lips (Fig. 8). Rapid viral tests and the later results of viral, fungal, and bacterial cultures did not reveal infection. Serum concentrations of methotrexate and metabolites were not done. Paraneoplastic pemphigus or a drug eruption were suspected. Biopsies of each area revealed interface dermatitis with necrotic keratinocytes with an infiltrate of perivascular lymphocytes. Biopsies of each area for direct immunofluorescence revealed no autoantibodies. The patient was discharged and it was decided to continue chemotherapy without methotrexate. The eruption resolved within 2 weeks.
|
|
| Figure 7 | Figure 8 |
|---|---|
|
Figure 7. Erosions and hemorrhagic bullae of Patient 3 Figure 8. Lip erosions in Patient 3 |
|
Discussion
Methotrexate is associated with many types of eruptions (see Table 1). The three eruptions reported here are probably linked to the administration of methotrexate because they each score a 5 (probable adverse drug reaction) on the Naranjo causality scale for adverse drug reactions (see Table 2).
Some of these cutaneous side effects are hypersensitivity reactions similar to the reactions any medication or antigen can induce. Others skin eruptions, in particular those following high dose methotrexate treatment, appear to result from cytotoxic-T lymphocytes and mononuclear cells that induce apoptosis in keratinocytes expressing drug-derived antigens at their surfaces, which is unsurprising because methotrexate can induce apoptosis [7, 8]. The range of manifestations of methotrexate-induced cutaneous toxicity is, however, notable and requires explication. The patients in this series manifested with interface dermatitis with necrotic keratinocytes or erosions and bullae and with eruptions that affect the back, knees, oral and vulvar mucosa, and acral skin.
The mucosal erosions that manifested in Patients 2 and 3 have a clear basis. Regions of rapid proliferation, such as the oral lining mucosa, show a greater frequency of ulceration than masticatory mucosa or body skin with chemotherapy treatment [9, 10, 11]. Thus, these mucosal eruptions appear to be induced by toxic effects of methotrexate on rapidly dividing cells.
Methotrexate's toxic effects on the hands likely have more complex mechanisms. These effects perhaps result in part from direct toxic effect to the acral epidermis from high concentrations of chemotherapy agents in areas that are vascular cul-de-sacs [12]. They are probably also underlined by the more rapid epidermal turnover in acral areas as opposed to thorax skin [13]. This correlates with the fact that the mitotic index correlates significantly with epithelial thickness, with the thicker regions showing a higher rate of proliferation [14, 15].
The interface dermatitis found on the back and knees of Patient 1 is harder to understand. Possible explanations for the interface dermatitis on the back and knees in Patient 1 include the high dose of methotrexate, the increased epidermal thickness of the areas involved with concomitant increased epidermal proliferation (albeit to a lesser extent then acral or mucosal skin), the patient's dependent position on his back during methotrexate administration, and the increased prevalence and extent of interface dermatitis in eruptions in patients with the acquired immunodeficiency syndrome [16]. The rippled almost livedoid appearance of this eruption on the back might have some basis in impairment of the patients cutaneous vasculature by methotrexate.
Treatment of methotrexate reactions
A variety of treatments have been proposed to treat toxic reactions or overdoses from methotrexate. Standard protocols involve the use of prednisone and antihistamines. A promising avenue is the bacterial enzyme carboxypeptidase G2 (CPDG2), which rapidly hydrolyzes methotrexate to inactive metabolites. Carboxypeptidase G2 has been used to reverse intrathecal overdoses of methotrexate [24]. The use of leucovorin (levogyrus folinic acid equivalent to double doses of the racemic product) is also commonly used even in high dose e.g., intravenously at a dose of 100 mg every 3 hours for 24 hours, and every 6 hours in the following 24 hours. Taurine protects against methotrexate-induced toxicity and inhibits leukocyte death [25].
Kohli et al. developed a high-dose MTX re-administration protocol based on a modified, prolonged carboplatin desensitization protocol. Over 1.5 hours, 1/1000 of the total intravenous dose was administered followed by 1/100 over 1.5 hours, 1/10 over 6 hours, and the rest of the full dose over 24 hours. Methotrexate re-administration was successfully tolerated on three occasions in a 17-year-old male patient with T-cell acute lymphoblastic lymphoma and a history of urticarial reactions to MTX [26].
Conclusion
Methotrexate causes a variety of eruptions in the skin. Just as the precise effects of methotrexate's mechanisms that facilitate amelioration of inflammatory skin diseases are not fully understood, the basis for its induction of cutaneous side effect remains to be fully defined. Some are likely the result of hypersensitivity reactions and others the result of apoptosis. In this case the eruptions were likely related to cytotoxic effects on keratinocytes and perhaps endothelial cells that induced cellular apoptosis. These apoptosis-related cytotoxic reactions should be recognized as a defined sub-category of eruptions induced by methotrexate.
References
1. http://www.rxlist.com/cgi/generic/mtx.htm (accessed November 10, 2005).2. Haustein UF, Rytter M. Methotrexate in psoriasis: 26 years' experience with low-dose long-term treatment. J Eur Acad Dermatol Venereol. 2000;14:382-8.
3. Plantin P, Saraux A, Guillet G. Methotrexate in dermatology: current aspects. Ann Dermatol Venereol. 1989;116:109-15.
4. Zachariae H. Methotrexate side-effects. Br J Dermatol. 1990;122 Suppl 36:127-33.
5. Olsen EA. The pharmacology of methotrexate. J Am Acad Dermatol. 1991;25:306-18.
6. Harrison PV. Methotrexate-induced epidermal necrosis. Br J Dermatol. 1987;116:867-9.
7. Heenen M, Laporte M, Noel JC, de Graef C. Methotrexate induces apoptotic cell death in human keratinocytes. Arch Dermatol Res. 1998;290:240-5.
8. Bell R, Sullivan JR, Burdon JG, Sinclair R. Toxic rash associated with high dose methotrexate therapy. Clin Exp Pharmacol Physiol Suppl. 1979;5:57-61.
9. Squier CA. Oral complications of cancer therapies. Mucosal alterations. NCI Monogr. 1990;(9):169-72.
10. Squier CA, Kremer MJ. Biology of oral mucosa and esophagus. J Natl Cancer Inst Monogr. 2001;(29):7-15.
11. Gibbs S, Ponec M.Intrinsic regulation of differentiation markers in human epidermis, hard palate and buccal mucosa. Arch Oral Biol. 2000;45:149-58.
12. Reynaert H, De Coninck A, Neven AM, Van Camp B, Schots R. Chemotherapy-induced acral erythema and acute graft-versus-host disease after allogeneic bone marrow transplantation. Bone Marrow Transplant. 1992;10:185-7.
13. Morris GM, Hamlet R, Hopewell JW. The cell kinetics of the epidermis and follicular epithelium of the rat: variations with age and body site. Cell Tissue Kinet. 1989;22:213-22.
14. Johnson GK, Squier CA, Johnson WT, Todd GL. Blood flow and epithelial thickness in different regions of feline oral mucosa and skin. J Oral Pathol. 1987;16:317-21.
15. Huang IT, Lin WM, Shun CT, Hsieh ST. Influence of cutaneous nerves on keratinocyte proliferation and epidermal thickness in mice. Neuroscience. 1999;94:965-73.
16. Patatanian E, Thompson DF. Rico MJ, Kory WP, Gould EW, Penneys NS. Interface dermatitis in patients with the acquired immunodeficiency syndrome. J Am Acad Dermatol. 1987;16:1209-18.
17. Kharfan Dabaja MA, Morgensztern D, Markoe AM, Bartlett-Pandite L. Radiation recall dermatitis induced by methotrexate in a patient with Hodgkin's disease. Am J Clin Oncol. 2000;23:531-3.
18. Yang CH, Yang LJ, Jaing TH, Chan HL. Toxic epidermal necrolysis following combination of methotrexate and trimethoprim-sulfamethoxazole. Int J Dermatol. 2000;39:621-3.
19. Goerttler E, Kutzner H, Peter HH, Requena L. Methotrexate-induced papular eruption in patients with rheumatic diseases: a distinctive adverse cutaneous reaction produced by methotrexate in patients with collagen vascular diseases. J Am Acad Dermatol. 1999;40(5 Pt 1):702-7.
20. Halevy S, Giryes H, Avinoach I, Livni E, Sukenik S. Leukocytoclastic vasculitis induced by low-dose methotrexate: in vitro evidence for an immunologic mechanism. J Eur Acad Dermatol Venereol. 1998;10:81-5.
21. Simonart T, Durez P, Margaux J, Van Geertruyden J, Goldschmidt D, Parent D. Cutaneous necrotizing vasculitis after low dose methotrexate therapy for rheumatoid arthritis: a possible manifestation of methotrexate hypersensitivity. Clin Rheumatol. 1997;16:623-5.
22. Martins da Cunha AC, Rappersberger K, Gadner H. Toxic skin reaction restricted to palms and soles after high-dose methotrexate. Pediatr Hematol Oncol. 1991;8:277-80.
23. Straka M, Zeringue E, Goldman M. A rare drug reaction to methotrexate after treatment for ectopic pregnancy. Obstet Gynecol. 2004;103(5 Pt 2):1047-8.
24. Widemann BC, Balis FM, Shalabi A, Boron M, O'Brien M, Cole DE, Jayaprakash N, Ivy P, Castle V, Muraszko K, Moertel CL, Trueworthy R, Hermann RC, Moussa A, Hinton S, Reaman G, Poplack D, Adamson PC. Treatment of accidental intrathecal methotrexate overdose with intrathecal carboxypeptidase G2. J Natl Cancer Inst. 2004;96:1557-9.
25. Cetiner M, Sener G, Sehirli AO, Eksioglu-Demiralp E, Ercan F, Sirvanci S, Gedik N, Akpulat S, Tecimer T, Yegen BC. Taurine protects against methotrexate-induced toxicity and inhibits leukocyte death.Toxicol Appl Pharmacol. 2005;209:39-50.
26. Kohli A, Ferencz TM, Calderon JG. Readministration of high-dose methotrexate in a patient with suspected immediate hypersensitivity and T-cell acute lymphoblastic lymphoma. Allergy Asthma Proc. 2004;25:249-52.
27. Arevalo-Lopez A. Acute methotrexate toxicity in psoriasis. Gac Med Mex. 1999;135:513-6.
28. Pearce HP, Wilson BB. Erosion of psoriatic plaques: an early sign of methotrexate toxicity. J Am Acad Dermatol. 1996;35:835-8.
© 2006 Dermatology Online Journal

