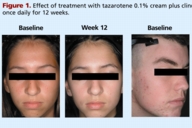
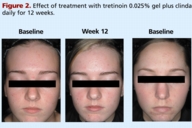
Tazarotene 0.1 percent cream plus clindamycin 1 percent gel versus tretinoin 0.025 percent gel plus clindamycin 1 percent gel in the treatment of facial acne vulgaris
Published Web Location
https://doi.org/10.5070/D30n81z2h4Main Content
Tazarotene 0.1 percent cream plus clindamycin 1 percent gel versus tretinoin 0.025 percent gel plus clindamycin 1 percent
gel in the treatment of facial acne vulgaris
Emil Tanghetti MD1, Sunil Dhawan MD2, Helen Torok MD3, Leon Kircik MD4
Dermatology Online Journal 13(3): 1
1. Center for Dermatology and Laser Surgery, Sacramento, CA. et@mgci.com2. Center for Dermatology Medical, Cosmetic, and Laser
Surgery, Inc., Fremont 3. Trillium Creek Dermatology and Surgery, Medina, OH, 4Physicians Skin Care PLLC, Louisville, KYAbstract
Topical retinoids are the cornerstone of therapy for acne vulgaris. Nevertheless, the adjunctive use of other anti-acne agents can help enhance the efficacy of topical retinoids still further. Given that tazarotene 0.1 percent gel has previously shown significantly greater efficacy than tretinoin 0.025 percent gel, it is likely that tazarotene plus clindamycin offers superior efficacy to tretinoin plus clindamycin, which has recently become available as a combination product. A total of 150 patients with facial acne vulgaris were randomly assigned to receive either tazarotene 0.1 percent cream plus clindamycin 1 percent gel, or tretinoin 0.025 percent gel plus clindamycin 1 percent gel. Each medication was applied once daily in the evening (clindamycin followed by the retinoid 5-10 minutes later) for up to 12 weeks. At week 12, the reduction from baseline in lesion counts was greater with tazarotene/clindamycin than tretinoin/clindamycin for both the non-inflammatory lesion count (71% vs. 52%, p≤.01) and the inflammatory lesion count (77% vs. 67%, P=.053). Tazarotene/clindamycin also resulted in a significantly higher incidence of patients achieving ≥ 50 percent global improvement (incidence of 88% vs. 75% at week 12; p≤.05). Both regimens were similarly well tolerated. In the treatment of facial acne vulgaris, tazarotene plus clindamycin offers significantly greater efficacy than tretinoin plus clindamycin and has comparable tolerability.
Introduction
Topical retinoids are the cornerstone of therapy for acne vulgaris as they inhibit the development of microcomedones—the precursors for all other acne lesions, both inflammatory and non-inflammatory. Nevertheless, the adjunctive use of anti-acne agents with a complementary mechanism of action can help enhance the efficacy of topical retinoid therapy still further. For example, using clindamycin in conjunction with tazarotene or tretinoin has been shown to offer significantly greater efficacy than either topical retinoid alone [1, 2, 3, 4]. The adjunctive use of clindamycin has also been shown to help minimize the potential for tolerability issues with topical retinoid therapy [1, 2].
Although the adjunctive use of a topical antibiotic can be a valuable strategy for maximizing efficacy in the short-term, experts advise that such treatment should generally not be used for more than a few weeks in order to minimize the risk of bacterial resistance developing [5]. The consensus opinion is that topical antibiotic therapy should be discontinued once inflammatory lesions are improved or after 6-8 weeks if no improvement has been observed [5]. If this is not possible, then benzoyl peroxide should be used in conjunction with the antibiotic as this reduces the potential for bacterial resistance. Therefore, although topical antibiotics can be a useful adjunct to therapy, they are appropriate for short-term use rather than long-term use. As a result, combination products incorporating a retinoid with an antibiotic in the same formulation have only limited clinical utility and are appropriate only for short-term treatment.
The only such product currently available in the US combines tretinoin with clindamycin. However, tazarotene 0.1 percent gel has previously been demonstrated to offer significantly greater efficacy than tretinoin 0.025 percent gel (as well as tretinoin 0.1 percent microsponge and adapalene 0.1 percent gel) [6, 7, 8] and therefore it is likely that tazarotene plus clindamycin (applied separately) would offer superior efficacy to tretinoin plus clindamycin (applied either separately or as a combination product). A study has been performed to evaluate this hypothesis.
Methods
Patients
Patients with facial acne vulgaris were eligible for enrollment in this multicenter, randomized, investigator-blind, parallel-group study if they were at least 12 years old and had 15-60 papules plus pustules, 10-100 comedones, and no more than 2 nodulocystic lesions (with a diameter no more than 5 mm).
The following washout periods were required before entry into the study-14 days for topical antibiotics and anti-acne medications, 30 days for systemic antibiotics and investigational drugs, 12 weeks for estrogens/birth control pills if used for less than 12 weeks before entering the study, and 12 months for oral retinoids.
Exclusion criteria included: known resistance to oral antibiotics; known hypersensitivity to lincomycin; history of enteritis; recent alcohol or drug abuse; any skin disorder that might interfere with the diagnosis or evaluation of acne vulgaris; any uncontrolled systemic disease; any cosmetic or surgical procedures complementary to the treatment of acne in the preceding 15 days; participation in an investigational drug study in the preceding 30 days; pregnancy; breastfeeding; and not practicing a reliable method of contraception.
This study was approved by the appropriate institutional review boards and conducted in accordance with Good Clinical Practice guidelines. All patients (and guardians where necessary) were required to give signed consent.
Treatment regimen
Patients were randomly assigned to receive tazarotene 0.1 percent cream plus clindamycin 1 percent gel, or tretinoin 0.025 percent gel plus clindamycin 1 percent gel. All medications were applied once daily in the evening after facial cleansing (with Cetaphil® Gentle Skin Cleanser, Galderma Laboratories, LP). Clindamycin was applied first and the retinoid was applied 5-10 minutes later (Table 1). Treatment was continued for up to 12 weeks and patients were evaluated every 4 weeks. A hydrating cream was provided (M.D. Forté® Replenish Hydrating Cream, Allergan, Inc.) and could be used as needed but no other lotions, creams, medicated powders, or solutions were allowed to be used on the face. Patients were requested to avoid excessive exposure to UV light.
Outcome measures
The investigators evaluated the patients every 4 weeks in terms of the non-inflammatory lesion count (open plus closed comedones), inflammatory lesion count (papules plus pustules), overall disease severity, global response to treatment, peeling, dryness, erythema, burning, pruritus, and perception of oiliness (Table 2). Investigators were provided with a photographic guide to grades 2-6 on the overall disease severity scale to aid in the assessment of this parameter.
Statistical analyses
It was calculated that a total of 150 subjects (75 in each group) would need to be enrolled in order for the study to detect a clinically significant between-group difference of 15 percent in the percent reduction in non-inflammatory lesion count at week 12. This assumed a standard deviation of 30 percent in both groups, a two-sided test, an α of .05, a power of 0.8, and a drop-out rate of 15 percent.
Statistical analyses of the study data were conducted on an intent-to-treat basis using two-sided tests. An α of .05 was considered to be statistically significant. The comparability of the two groups at baseline was evaluated using a chi-square test or Fisher's exact test for gender, race, and general appearance of the skin, and a t-test (or Wilcoxon rank sum test when the hypothesis of normality was rejected) for age and lesion counts. Between-group differences in the reduction in lesion counts were evaluated using an ANCOVA technique with the baseline value as the covariate (provided the necessary assumptions were satisfied), a rank ANCOVA (if the necessary assumptions for ANCOVA were not satisfied), or a t-test (or Wilcoxon rank sum test when the hypothesis of normality was rejected) if the baseline value did not have a significant effect in the model. Between-group differences in the reduction in overall disease severity score and the mean score for dryness, erythema, peeling, burning, pruritus, and perception of oiliness were evaluated using a Wilcoxon rank sum test. A chi-square test or Fisher's exact test was used to evaluate the between-group difference in the incidence of ≥ 50 percent or
75 percent global improvement and the incidence of adverse events at least probably related to treatment.
Results
Patients
Of 150 patients enrolled, 135 (90%) completed. One (1%) patient in each group discontinued due to an adverse event (dryness in a patient treated with tazarotene/clindamycin, and redness and irritation in a patient treated with tretinoin/clindamycin). In addition, a total of 11 patients were lost to follow-up, 1 withdrew their consent, and 1 discontinued for other unspecified reasons.
The patients had a mean age of 21 years (range, 12-58 years) and the majority were female (59%) and Caucasian (65% Caucasian, 11% Asian, 9% black, 7% Hispanic, 7% other). The patients considered the general appearance of their skin to be normal to oily (40%), mixed oily/dry (24%), normal (13%), normal to dry (11%), oily (9%), or dry (3%). At baseline, they had a mean of 40 open plus closed comedones and 25 papules plus pustules.
There were no significant between-group differences in the patients' age, gender, race, general appearance of skin, or lesion counts at baseline.
Efficacy
The tazarotene/clindamycin regimen was more effective in reducing lesion counts and in achieving overall improvements in acne than the tretinoin/clindamycin regimen.
 |  |
| Figure 3 | Figure 4 |
|---|---|
| Figure 3. Reduction in open plus closed comedo count Figure 4. Reduction in papule plus pustule count (CLICK for full size images) | |
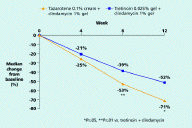
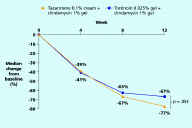
With regard to lesion counts, tazarotene/clindamycin achieved greater reductions than tretinoin/clindamycin in the non-inflammatory lesion count (71% vs. 52% at week 12, p≤.05) and the inflammatory lesion count (77% vs. 67% at week 12, p=.053 (NS)). The tazarotene regimen also resulted in a more rapid reduction in the non-inflammatory lesion count, achieving a significantly greater reduction than tretinoin/clindamycin as early as week 8 (53% vs. 39%, p≤.01).
 |  |
| Figure 5 | Figure 6 |
|---|---|
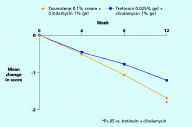
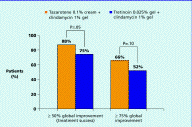
| Figure 5. Reduction in overall disease severity score Figure 6. Incidence of ≥ 50 percent or ≥ 75 percent global improvement at week 12 (CLICK for full size images) | |
With regard to overall improvement in acne, tazarotene/clindamycin was significantly more effective than tretinoin/clindamycin in reducing the overall disease severity score (by 1.7 vs. 1.2 at week 12, p≤.05) (Figure 5). In addition, a greater proportion of patients achieved ≥ 50 percent global improvement and ≥ 75 percent global improvement with tazarotene/clindamycin than with tretinoin/clindamycin—88 percent vs. 75 percent (p≤.05) and 66 percent vs. 52 percent (p=.10 (NS)), respectively, at week 12 (Figure 6).
Tolerability
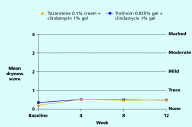
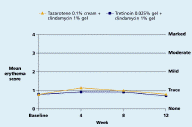
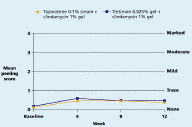
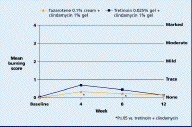
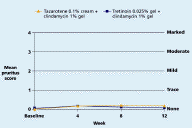
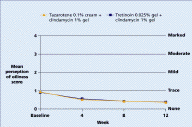
Both regimens were generally well tolerated with mean levels of dryness, erythema, peeling, burning, pruritus, and perception of oiliness being trace or less throughout the study in both groups.
 |  |
| Figure 7 | Figure 8 |
|---|---|
| Figure 7. Mean dryness score Figure 8. Mean erythema score (CLICK for full size images) | |
 |  |
| Figure 9 | Figure 10 |
|---|---|
| Figure 9. Mean peeling score Figure 10. Mean burning score (CLICK for full size images) | |
 |  |
| Figure 11 | Figure 12 |
|---|---|
| Figure 11. Mean pruritus score Figure 12. Mean perception of oiliness score (CLICK for full size images) | |
There was no significant between-group difference in the incidence of adverse events considered at least probably related to treatment—4 percent (3/75) in the tazarotene/clindamycin group (facial sunburn; dryness; red blotches on neck, itchy face, and possible cold symptoms) and 3 percent (2/75) in the tretinoin/clindamycin group (dryness and burning; itching on lower cheeks and jaw line). No serious adverse events were reported.
Conclusions
Tazarotene plus clindamycin offers significantly greater efficacy than tretinoin plus clindamycin when used once daily to treat facial acne vulgaris. This confirms the results of previous multicenter, double-blind, randomized trials that demonstrated significantly greater efficacy with tazarotene 0.1 percent gel than tretinoin 0.025 percent gel [6]. The tazarotene/clindamycin and tretinoin/clindamycin regimens in the study reported here were similarly well tolerated.
It may be wondered whether the efficacy of the medications might be compromised by applying them within a few minutes of each other—as might occur, for example, if the active agent in one formulation was not stable in the presence of the other formulation. However, the layering of the products in this study did not appear to compromise efficacy—the reductions in inflammatory and non-inflammatory lesion counts with tretinoin plus clindamycin were comparable to those that have been reported previously in studies using tretinoin/clindamycin combination products [9, 10].
The results of this study have important implications in light of the recent introduction in the US of a combination product incorporating both tretinoin 0.025 percent and clindamycin 1 percent. Not only is such a product appropriate for short-term therapy only (due to the need to minimize the risk of bacterial resistance) but it likely offers significantly inferior efficacy to tazarotene 0.1 percent cream and clindamycin 1 percent gel. Therefore, it would be more effective—and also perhaps easier for patients to understand and remain compliant with their treatment—if they applied both tazarotene 0.1 percent cream and clindamycin 1 percent gel from the outset of therapy and simply discontinued the clindamycin after the first few weeks of therapy once their inflammatory lesions had improved. This may be a simpler transition to maintenance therapy than requiring patients to discontinue their original combination product and then start therapy with a new product (i.e. topical retinoid monotherapy). If there is a need during maintenance therapy to achieve greater efficacy than can be obtained with a topical retinoid alone, it would be rational to add both benzoyl peroxide and clindamycin to the existing retinoid regimen. This is because a combination benzoyl peroxide/clindamycin product (a ready-to-dispense formulation containing two emollients) has previously been demonstrated to enhance the efficacy of tazarotene monotherapy and, possibly, to also offer a tolerability advantage [11]. Using a benzoyl peroxide and clindamycin combination is preferable to adding clindamycin alone (which, without the presence of benzoyl peroxide, would increase the risk of bacterial resistance developing) or adding benzoyl peroxide alone (which has been shown in one study not to enhance the efficacy of tazarotene monotherapy [1]).
Acknowledgment: This study was supported by a grant from Allergan, Inc., Irvine, CA 92612
References
1. Draelos ZD, Tanghetti EA, Tazarotene Combination Leads to Efficacious Acne Results (CLEAR) Trial Study Group. Optimizing the use of tazarotene for the treatment of facial acne vulgaris through combination therapy. Cutis. 2002 Feb;69(2 Suppl):20-9. PubMed2. Richter JR, Forstrom LR, Kiistala UO, Jung EG. Efficacy of the fixed 1.2% clindamycin phosphate, 0.025% tretinoin gel formulation (Velac) and a proprietary 0.025% tretinoin gel formulation (Aberela) in the topical control of facial acne. J Eur Acad Dermatol Venereol. 1998 Nov;11(3):227-33. PubMed
3. Cambazard F. Clinical efficacy of Velac, a new tretinoin and clindamycin phosphate gel in acne vulgaris. J Eur Acad Dermatol Venereol. 1998 Sep;11 Suppl 1:S20-7; discussion S28-9. PubMed
4. Leyden JJ, Krochmal L, Yaroshinsky A. Two randomized, double-blind, controlled trials of 2219 subjects to compare the combination clindamycin/tretinoin hydrogel with each agent alone and vehicle for the treatment of acne vulgaris. J Am Acad Dermatol. 2006 Jan;54(1):73-81. Epub 2005 Nov 28. PubMed
5. Gollnick H, Cunliffe W, Berson D, et al. Management of acne: a report from a Global Alliance to Improve Outcomes in Acne. J Am Acad Dermatol. 2003 Jul;49(1 Suppl):S1-37. PubMed
6. Webster GF, Berson D, Stein LF, Fivenson DP, Tanghetti EA, Ling M. Efficacy and tolerability of once-daily tazarotene 0.1% gel versus once-daily tretinoin 0.025% gel in the treatment of facial acne vulgaris: a randomized trial. Cutis. 2001 Jun;67(6 Suppl):4-9. PubMed
7. Leyden JJ, Tanghetti EA, Miller B, Ling M, Berson D, Lee J. Once-daily tazarotene 0.1 % gel versus once-daily tretinoin 0.1 % microsponge gel for the treatment of facial acne vulgaris: a double-blind randomized trial. Cutis. 2002 Feb;69(2 Suppl):12-9. PubMed
8. Webster GF, Guenther L, Poulin YP, Solomon BA, Loven K, Lee J. A multicenter, double-blind, randomized comparison study of the efficacy and tolerability of once-daily tazarotene 0.1% gel and adapalene 0.1% gel for the treatment of facial acne vulgaris. Cutis. 2002 Feb;69(2 Suppl):4-11. PubMed
9. ZianaTM prescribing information. Available at: http://www.medicis.com/products/pi/pi_ziana.pdf. Accessed March 16, 2007.
10. Leyden JJ, Krochmal L, Yaroshinsky A. Two randomized, double-blind, controlled trials of 2219 subjects to compare the combination clindamycin/tretinoin hydrogel with each agent alone and vehicle for the treatment of acne vulgaris. J Am Acad Dermatol. 2006 Jan;54(1):73-81. Epub 2005 Nov 28. PubMed
11. Tanghetti E, Abramovits W, Solomon B, Loven K, Shalita A. Tazarotene versus tazarotene plus clindamycin/benzoyl peroxide in the treatment of acne vulgaris: a multicenter, double-blind, randomized parallel-group trial. J Drugs Dermatol. 2006 Mar;5(3):256-61. PubMed
© 2007 Dermatology Online Journal