Zinc and skin: A brief summary
Published Web Location
https://doi.org/10.5070/D30m58m2w9Main Content
Zinc and skin: A brief summary
Piyush Kumar MD, Niharika Ranjan Lal MBBS, Ashim Kumar Mondal MBBS, Avijit Mondal MD, Ramesh C Gharami MD, Arunasis Maiti
MD
Dermatology Online Journal 18 (3): 1
Medical College & Hospital, Kolkata, IndiaAbstract
Zinc is an essential trace element that is an integral component of many metallo-enzymes in the body and thus serves many biological functions. The clinical presentation of zinc deficiency varies and depends on serum zinc level. Whereas a significantly low serum zinc level results in clinical features similar to acrodermatitis enteropathica, mild hypozincemia presents with a less characteristic appearance; hence it may be underdiagnosed. Recognition of various cutaneous lesions is required for suspecting and identifying cases of zinc deficiency. Although many laboratory tests are useful, therapeutic response in suspected cases remains the gold standard of diagnosis. Serum zinc estimation alone is not very reliable because disease activity may not necessarily correlate with serum zinc level. Zinc supplementation results in a rapid response and the skin lesions heal without permanent sequelae. However, pigmentary alterations may persist longer. Predisposing factors should be identified and corrected. This brief review summarizes the identification and management of clinical zinc deficiency.
Acrodermatitis enteropathica (AE) is a well-recognized entity caused by an inherited defect in zinc absorption leading to hypozincemia. Acquired hypozincemia has also been described; the clinical presentation varies depending on whether the serum zinc level is significantly low or mildly low. A significantly low serum zinc level results in periorificial erosive dermatitis, mimicking AE. A mildly low serum zinc level presents with less characteristic psoriasiform skin lesions [1]. Because of the less characteristic appearance, mild forms may be under diagnosed [2]. However, zinc deficiency is a much more complex process and the clinical presentation does not always correlate with the zinc level, as described below. Nonetheless, recognition of cutaneous lesions related to zinc deficiency is important for several reasons. First of all, they are the most visible among all manifestations and are specific enough to allow diagnosis with reasonable certainty. Secondly, the condition responds well to zinc supplementation. All the manifestations, if treated in time, resolve without sequelae. However, if not treated, it may result in various long-term morbidties. Hence, this condition requires early recognition and treatment to prevent long-term deleterious effects on overall health. Herein we review different clinical manifestations of zinc deficiency and its management.
The biological role of zinc in human beings has long been recognized, but clinical zinc deficiency in humans was identified much later [3]. In short, zinc is an essential trace element that is an integral component of many metallo-enzymes in the body and thus serves many biological functions. Zinc stabilizes the cell membranes and protects their integrity by reducing the formation of free radicals and by the prevention of lipid peroxidation. Zinc is required for immune system function. It aids in protein synthesis, cell reproduction, and wound healing, and plays a major role in fertility and conception [3]. Zinc supplementation has found many usages in medicine including reducing the duration of malaria and the severity of respiratory and diarrheal diseases [3].
 |
| Figure 1 |
|---|
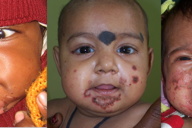
| Figure 1. Perioral erosive dermatitis in “acute” zinc deficiency. The severity of erosion, crusting, and erythema may vary. |
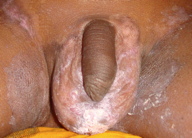
Zinc deficiency may be either inherited or acquired. Both forms have similar clinical manifestations. Acrodermatitis enteropathica (AE), the inherited form, was first described by Danbolt and Closs in 1943. However, the cause was unknown and the disease was often fatal [4]. The link between AE and zinc deficiency was not realized until 30 years after the initial clinical description. Genetic study in consanguineous Jordanian and Egyptian kindreds with AE localized the genetic defect to 8q24 [5]. The defective gene was identified as SLC39A4 (Solute carrier family 39, member 4), which encodes a protein called human zinc/iron-regulated transporter-like protein (hZIP4) [6, 7]. Acrodermatitis enteropathica classically presents during infancy on weaning from breast milk to formula or cereal; these have lower zinc bioavailability than breast milk. The condition is characterized by a triad of dermatitis, diarrhea, and alopecia [1]. However, only 20 percent of patients present with all three components at a given time [2]. Patients initially present with perioral erosive dermatitis and perleche, that progresses to involve the face, acral parts, and diaper area (Figures 1, 2, and 3). Palmar erythema, sometimes with annular scales, may be present. The predominant extracutaneous feature is diarrhea, which is the most variable manifestation [2]. It may be intermittent or totally absent. It may result in decreased zinc absorption and may further aggravate the condition, a vicious cycle [2]. Photosensitivity is a frequent association. Hence, if any baby presents with periorificial erosive dermatitis along with alopecia, diarrhea and/or photosensitivity, zinc deficiency should be considered. At times, patients present with persistent cutaneous infections (e.g., candidiasis, paronychia, blepharitis, and conjunctivitis), instead of characteristic cutaneous lesions [2].
 | 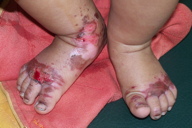 |
| Figure 2 | Figure 3 |
|---|---|
| Figure 2. Erosive dermatitis affecting the diaper area Figure 3. Erosion and scald-like erythema on the acral parts | |
Recently, hypozincemia in children has been classified into three types [8, 9]: Type I is characterized by an inherent defect in the absorption of zinc from the gut, i.e., classical AE. It is transmitted in an autosomal recessive manner. Type II occurs because of impaired secretion of zinc in breast milk. Recently, a missense mutation in the SLC30A2 gene (encoding ZnT-2 protein) has been identified as a cause for defective transfer of zinc from serum to breast milk. It is reported to have an autosomal recessive or sex linked inheritance [9]. The infant of such a mother can present even when exclusively breastfed. Type III develops in preterm infants who are put on prolonged parenteral alimentation deficient in zinc. Recognition of these types is important as type I presents usually during weaning.
Long standing zinc deficiency has been found to be associated with frequent infections, delayed wound healing, growth retardation, anorexia, anemia, photophobia, hypogonadism, delayed puberty, and altered mental status [2, 4].
Acquired zinc deficiency may result from states associated with inadequate intake, impaired absorption, increased demand, or increased excretion and is seen in pregnancy, lactation, extensive cutaneous burns, generalized exfoliative dermatoses, food faddism, parenteral nutrition, anorexia nervosa, and even excessive sweating. Some other conditions associated with acquired hypozincemia are intestinal malabsorption syndromes (inflammatory bowel disease), cystic fibrosis, alcoholism, HIV infection, malignancy, uremia, and chronic renal disease (especially nephrotic syndrome) [2, 10].
The cutaneous changes of acquired zinc deficiency are similar, usually milder than that of the inherited form [2]. It is especially true for conditions causing significant depletion of zinc levels [11]. The skin lesions develop over a few days and are mainly periorificial in location. The lesions are eczematous scaly plaques that can develop into vesicles, bullae, or pustules. The underlying skin has a “scald like” erythema, and may show fissuring [12]. Areas subjected to constant rubbing, like the side of the face, skin in contact with NG tubes, and heels in bed-ridden patients develop similar lesions [12]. Secondary bacterial and fungal infections are common. Angular cheilitis is a common early manifestation, followed by paronychia. In the absence of treatment, skin lesions become eroded and patients develop alopecia and photophobia [10].
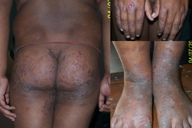 |  |
| Figure 4 | Figure 5 |
|---|---|
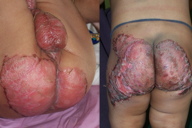
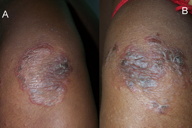
| Figure 4. Crusted erythematous plaques in “chronic” zinc deficiency in a case of nephrotic syndrome Figure 5. Annular (A) and psoriasiform (B) lesions in the same patient | |
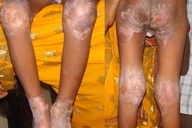
The skin lesions resulting from mild or moderate hypozincemia are less dramatic [1, 3, 4, 12]. In mild hypozincemia, the characteristic feature is a lack of inflammation and “scald like” erythema. The lesions are typically seen on areas subject to repeated pressure and trauma, such as elbows, knees, knuckles, malleolar regions of the ankles, and the sacral area. The cutaneous lesions may be psoriasiform plaques (in appearance and distribution), annular plaques with brown black crusts at advancing margins and scaling at the center, crusted plaques, or pigmented lichenoid lesions [12] (Figures 4, 5, 6, and 7). Vesicobullae, pustules, and erosions are occasional findings. There is decreased hair and nail growth. Diffuse thinning of the scalp hair may be seen. Hairs are brown and sometimes, white. Structural changes of hair, such as breakage, spearhead-like appearance, transverse striations, longitudinal splits, pseudo-monilithrix, and bayonet shape have been described. Nails may show inflammation of the cuticles, white transverse bands, thinning, Beau lines, and sometimes, dystrophy [12, 13]. Ocular involvement, in the form of photophobia, conjunctivitis, blepharitis, corneal dystrophy, and rarely cataract, may be seen [14, 15].
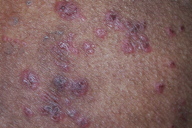 | 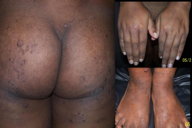 |
| Figure 6 | Figure 7 |
|---|---|
| Figure 6. Close up view of the lesions Figure 7. Follow up picture of a patient of “chronic” zinc deficiency receiving zinc supplementation for 1 month | |
Apoptosis has been considered as the pathological basis of cutaneous changes in zinc deficiency [16]. Wilson et al have shown no effects of zinc deficiency on cell proliferation, changes in the protein levels or intracellular localization of cytokeratins, or changes in the cell-cell adhesion protein, E-cadherin. Interestingly, they have shown that apoptosis can be induced rapidly if the zinc level is depleted to a dramatically low level. However, if the zinc level is not that low, apoptosis is slower in onset [16]. The authors believe that this observation may explain different clinical presentations seen with different zinc levels.
The differential diagnoses include biotin deficiency, essential fatty acid (EFA) deficiency, vitamin B2 (riboflavin) deficiency, necrolytic migratory erythema (NME), pseudoglucagonoma syndrome, atopic dermatitis, psoriasis, and candidiasis [1, 2, 11]. Biotin deficiency is characterized by neurological features like hyperaesthesia, myoclonic seizures, hypotonia, ataxia, and hearing loss, apart from cutaneous features similar to hypozincemia. The diagnosis can be established by serum biotin estimation and increased urinary excretion of organic acid (3-hydroxyisovaleric acid). Essential fatty acid deficiency is rare and can be diagnosed by demonstrating decreased plasma levels of linoleic acid and arachidonic acid and an increased level of eicosatrienoic acid. Riboflavin deficiency is characterized by prominent ocular involvement (oculo-orogenital syndrome) and elevated activation coefficient of erythrocyte gluthathione reductase establishes the diagnosis. Necrolytic migratory erythema develops in older patients with glucagon secreting pancreatic tumors and is characterized by a dramatic elevation of the glucagon level, often over 1,000 pg/ml. Pseudoglucagonoma syndrome presents similarly, but is not associated with pancreatic tumor and hence, does not show such an elevated glucagon level. Liver diseases (alcoholic liver diseases, hemochromatosis, and hepatitis B virus infection), pancreatitis, celiac sprue, inflammatory bowel disease, and pellagra have been identified as the underlying conditions [1, 2, 11].
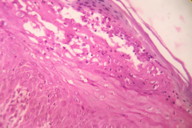 |
| Figure 8 |
|---|
| Figure 8. Histopathology showing parakeratosis, necrosis of upper layers of epidermis and scattered dyskeratotic cells (H&E, x400) |
Hypozincemia should be included in differential diagnosis of periorificial erosive dermatitis. The diagnosis of mild hypozincemia requires a high index of suspicion. The determination of a low plasma zinc level is diagnostic. Normal plasma zinc level is 70-250 μg/dl and many patients have plasma levels less than 50 μg/dl [1, 3]. However, mild deficiency (40-60 μg/dl) also can manifest clinically, albeit in a different manner [1]. Hypoalbuminea, hemolyzed samples, and contamination may give erroneous values for zinc [1, 17]. Recommendations to use zinc-free vacuum tubes and stainless steel needles, avoid contact with rubber stoppers (they contain zinc), avoid hemolysis, separate plasma or serum from cells within 45 min, and use anticoagulants that are low in zinc or zinc-free have been made. There is also a diurnal rhythm in plasma zinc concentration; a morning fasting sample is recommended for accurate results [17]. In conclusion, zinc estimation suffers from many limitations. Moreover, a few authors, Mack et al [18], Oh et al [19], and more recently, Rad et al [20], have described cases of AE with normal serum zinc levels. On the other hand, zinc concentrations can be low without zinc depletion in certain other circumstances such as acute infections [19]. Considering all these issues, the use of serum/plasma zinc measurement alone for arriving at a diagnosis is not advocated [21]. Zinc level estimation from urine or hair samples may be done. However, the urine zinc level varies considerably and hence, is not useful for diagnostic purposes. Similarly, hair zinc estimation is not free from limitations because the hair zinc level is not a good indicator of the acute state, rather it is an indicator of the chronic state. Moreover, it may be markedly depressed in mild zinc deficiency states and normal in cases of severe zinc deficiency (when hair growth is arrested). Therefore, it, does not correlate with body zinc stores or disease severity [19]. Serum alkaline phosphatase is a zinc dependent enzyme and may serve as surrogate marker of hypozincemia.
In cases in which zinc values are equivocal, histopathology may be of diagnostic help. The most relevant clue is the presence of “necrolysis” a term describing cytoplasmic pallor, vacuolization, ballooning degeneration, and subsequent confluent necrosis of keratinocytes in the upper part of the epidermis [10, 22, 23] (Figure 8). The affected keratinocytes often have pyknotic nuclei. Parakeratosis is present and is typically confluent rather than focal, usually associated with loss of the granular layer and dermal edema. Subcorneal vesicle formation in the pale areas may be observed. Associated neutrophilic crust is a variable finding. Scattered dyskeratotic keratinocytes are often present within all levels of the epidermis [10, 22]. The histological findings of pellagra and NME are indistinguishable from that of hypozincemia. Late or chronic lesions mimic psoriasis; necrolysis and pallor of keratinocytes are minimal or absent [10].
Clinical response to zinc administration (3-30 μmol zinc/kg per day for 5 days) is another important diagnostic tool [21]. If no clinical improvement occurs within a few days and the serum alkaline phosphatase remains stable, the patient is not deficient in zinc [24].
Zinc supplementation in a dose of 1-2 mg/kg/day is the standard therapy. In the hereditary form (type I), lifelong supplementation is required. In the acquired form, the duration of therapy will depend on etiology. Typically, clinical improvement is seen very rapidly, within days to weeks, before there is a significant change in serum zinc levels. There is rapid improvement of diarrhea within 24 hours and the erosive skin lesions heal within 1 to 2 weeks of zinc therapy without additional topical therapy [25]. However, it may take longer, depending on the degree of depletion of zinc [3] (Figures 9 and 10). Serum zinc and alkaline phosphatase will rise during therapy [26]. In the hereditary form, serum or plasma zinc levels and zinc-dependent enzyme levels (e.g., alkaline phosphatase) should be monitored every 3 to 6 months and the dose of zinc should be adjusted accordingly [9]. Overzealous treatment should be avoided because zinc supplementation can lower blood copper levels and large accidental overdoses may cause multisystem organ failure [4]. Side effects of zinc therapy include gastric irritation with nausea, vomiting, and gastric hemorrhage.
To conclude, awareness of cutaneous manifestations of zinc deficiency, especially those resulting from mildly depleted zinc levels is important. Classic AE and similar presentations are generally widely recognized. When suspected, various diagnostic modalities are available. However, these should be used with caution because zinc measurements suffer from many limitations. The clinical response to zinc supplementation remains the gold standard of diagnosis. Hence, in all suspected patients, a trial of zinc therapy should be undertaken because it may help in avoiding unnecessary investigations. Any predisposing condition, if present, should be identified and corrected.
References
1. Jen M, Shah KN, Yan AC. Cutaneous changes in nutritional diseases. In: Wolff K, Goldsmith LA, Katz SI et al., editors. Fitzpatrick’s Dermatology in General Medicine. 7th edn. New York: McGraw Hill; 2008. 1209-16.2. Schmuth M, Fritsch PO. Cutaneous changes in nutritional diseases. In Krutmann J, Humbert P, editors. Nutrition for healthy skin. 1st ed. Berlin: Springer-Verlag Berlin Heidelberg; 2011. 8-10.
3. Suchithra N, Sreejith P, Pappachan JM, George J, Rajakumari PK, Cheriyan G. Acrodermatitis enteropathica-like skin eruption in a case of short bowel syndrome following jejuno-transverse colon anastomosis. Dermatology Online Journal 2007;13(3):20. [PubMed]
4. Maverakis E, Fung MA, Lynch PJ, Draznin M, Michael DJ, Ruben B, et al. Acrodermatitis enteropathica and an overview of zinc metabolism. J Am Acad Dermatol 2007;56(1):116-24. [PubMed]
5. Wang K, Pugh EW, Griffen S, Doheny KF, Mostafa WZ, al-Aboosi MM, el Shanti H, Gitschier J. Homozygosity mapping places the Acrodermatitis enteropathica gene on chromosomal region 8q24.3. Am J Hum Genet 200;68(4):1055-60. [PubMed]
6. Kury S, Dreno B, Bezieau S, Giraudet S, Kharfi M, Kamoun R, Moisan JP. Identification of SLC39A4, a gene involved in acrodermatitis enteropathica. Nat Genet 2002;31(3):239-40. [PubMed]
7. Nakano A, Nakano H, Nomura K, Toyomaki Y, Hanada K. Novel SLC39A4 Mutations in Acrodermatitis Enteropathica. J Invest Dermatol 2003;120:963-6. [PubMed]
8. Sharma NL, Sharma RC, Gupta KR, Sharma RP, Mahajan VK. Hypozincemia in infancy. Indian J Dermatol Venereol Leprol 1985; 51: 256-60.
9. Murthy SC, Udagani MM, Badakali AV, Yelameli BC. Dermatology Online Journal 2010;16(6). [PubMed]
10. Montinari M, Parodi A, Rongioletti F. Acquired Nutritional deficiencies. In Rongioletti F, Smoller BR, editors. Clinical and pathological aspects of skin diseases in Endocrine, Metabolic, Nutritional and deposition disease. 1st ed. New York: Springer; 2010. 101-8.
11. Maldonado RR, Orozco-Covarrubias L. Nutritional diseases. In: Bolognia JL, Jorizzo JL, Rapini RP, editors. Dermatology. 2nd edn. Philadelphia: Mosby Elsevier; 2008. 669-71.
12. Seneviratne JKK. Cutaneous manifestations of zinc deficiency in children. Sri Lanka Journal of Child Health 2002;31:106-8.
13. Weismann K. Lines of Beau: possible markers of zinc deficiency. Acta Derm Venereol 1971;57:88-90. [PubMed]
14. Kaur S, Thami GP, Kanwar AJ. Acrodermatitis Enteropathica in a full-term breast-fed infant. Indian J Pediatr 2002; 69: 631-3. [PubMed]
15. Simsek E. Acrodermatitis Enteropathica. Turk J Med Sci 2001;31:573-4.
16. Wilson D, varigos G, ackland ML. Apoptosis may underlie the pathology of zinc-deficient skin. Immunology and Cell Biology 2006;84:28-37. [PubMed]
17. Mashhood AA. Role of correct dose of zinc sulphate in the treatment of acrodermatitis enteropathica in two siblings. Journal of Pakistan Association of Dermatologists 2007;17:116-21.
18. Mack D, Koletzko B, Cunnane S, Cutz E, Griffiths A. Acrodermatitis enteropathica with normal serum zinc levels: diagnostic value of small bowel biopsy and essential fatty acid determination. Gut 1989; 30(10):1426-9. [PubMed]
19. Oh K, Kim JH, Lee JE, Lim DH, Son BK. A case of acquired acrodermatitis enteropathica with a normal serum zinc level but a low level in the hair. Korean J Pediatr 2007;50:209-12.
20. Rad F, Yaghmaee R. Acrodermatitis enteropathica with a normal serum zinc level: a case report. Scientific Journal of Kurdistan University of Medical Sciences 2010;15(2):98-103.
21. Van Wouwe JP. Clinical and laboratory diagnosis of acrodermatitis enteropathica. Eur J Pediatr 1989;149(1):2-8. [PubMed]
22. Macgro C , Crowson AN, Dyrson M, Mihm M. Cutaneous Manifestations of Nutritional Deficiency States and Gastointestinal Disease. In: Elder DE, Elenitsas R, Johnson BL, Murphy GF, Xu X, editors. Lever’s histopathology of skin. 10th edition. Philadelphia: Lippincott Williams & Wilkins publishers; 2009. 409-10.
23. Jensen SL, McCuaig C, Zembowicz A, Hurt MA. Bullous lesions in acrodermatitis enteropathica delaying diagnosis of zinc deficiency: a report of two cases and review of the literature. J Cutan Pathol 2008;35(Suppl.1):1-13. [PubMed]
24. Arlette JP. Zinc and the skin. Pediatr Clin North Am 1983; 30:583-96 [PubMed]
25. Chue CD, Rajpar SF, Bhat J.An acrodermatitis enteropathica-like eruption secondary to acquired zinc deficiency in an exclusively breast-fed premature infant. Int J Dermatol 2008; 47: 372-3 [PubMed]
26. Weismann K. Zinc metabolism and the skin. In: Rook A, Savin J, eds. Recent Advances in Dermatology, Vol. 5. London: Churchill Livingstone, 1980: 109-29.
© 2012 Dermatology Online Journal