A case of bullous disease limited to the skin illustrates the spectrum of neoplasia induced autoimmunity
Published Web Location
https://doi.org/10.5070/D30jg6v69bMain Content
A case of bullous disease limited to the skin illustrates the spectrum of neoplasia induced autoimmunity
Raminder K Grover MD1, Craig P Stites MD2, Thomas N Helm MD3, Takashi Hashimoto MD4, Ernst H Beutner PhD1
Dermatology Online Journal 15 (7): 5
1. Beutner Laboratories and the Departments of Microbiology and Immunology and Dermatology, School of Medicine and Biomedical
Sciences, University at Buffalo, State University of New York, New York. rgrover2@buffalo.edu2. AR/OK Dermatology Center, Ft. Smith, Arkansas
3. Department of Dermatology, University at Buffalo, State University of New York
4. Department of Dematology, Kurume University School of Medicine, Kurume, Fukuoka, Japan
Abstract
Although the initial report of paraneoplastic pemphigus described individuals with mucocutaneous blistering disease, subsequent reports identified patients with lichen planus or graft versus host disease-like changes. We describe a patient with fatal autoimmune blistering disease with absence of mucous membrane lesions. The pattern of complement indirect immunofluoresence helped identify the prognosis prospectively. This case illustrates yet another presentation of the neoplasia-induced autoimmunity.
Introduction
Most cases of paraneoplastic pemphigus are characterized by severe and painful erosions of the mouth. Involvement of the pharynx, larynx, esophagus, eyes, nose, and genitalia may occur. The clinical, histological and immunological heterogeneity of this disease became apparent with reports of more cases since its first description [1-6]. Because multiple organs may be involved, the name paraneoplastic autoimmune multiorgan syndrome (PAMS) has been suggested [6]. Recent reports describe graft versus host disease-like changes occurring in the setting of thymoma [7] and we describe a patient with an atypical presentation of blistering disease, but with positive indirect immunoflouresence complement fixing antibodies. These cases illustrate that neoplasia-induced autoimmunity encompassed a broader spectrum than the original described cases of paraneoplastic pemphigus [1].
Case report
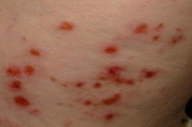
An 82-year-old woman presented with a three-day history of extremely pruritic, tense bullae of the antecubital and popliteal fossae, buttocks, and abdomen. She had a history of non- Hodgkin's lymphoma in remission for one year and had completed five courses of cyclophosphamide, vincristine, prednisone and rituximab therapy. The current medications included acetylsalicylic acid (81 mg) and multivitamins. Examination revealed urticarial plaques with tense bullae on the flexural aspect of the upper arms, extensor elbows, flexural knees, extensor knees, buttocks and lateral thighs (Fig. 1A). The head, neck, palms, and soles were spared. No ocular, oral or genital mucosal involvement was present but she had significant cervical, axillary and inguinal lymphadenopathy. Complete blood counts revealed pancytopenia and CT scan revealed numerous enlarged lymph nodes.
 |  |
| Figure 1A | Figure 1B |
|---|---|
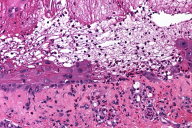
| Figure 1. Tense bullous lesions on the thighs and buttock at the time of presentation to the dermatology clinic (A) and histologic findings, perilesional biopsy (B) (H&E, x400) | |
Biopsy revealed vesicle formation with areas of subepidermal cleft formation and suprabasilar acantholysis. Necrotic keratinocytes and a patchy lymphohistiocytic inflammatory infiltrate were identified. Numerous eosinophils were noted within the inflammatory infiltrate. Mixed features of bullous pemphigoid, erythema multiforme and pemphigus vulgaris were evident (Fig. 1B).
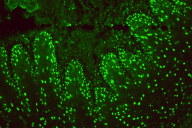 |
| Figure 2C |
|---|
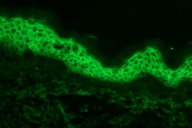
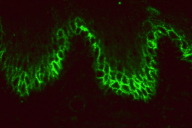
Direct immunofluorescenc studies on a perilesional skin biopsy revealed strong epidermal cell surface IgG and IgG1 (Figure 2A), but no IgG4 deposits, and focal C3 on the outer cytoplasm of basal cells. Indirect immunofluoresence tests for IgG and IgG4 antibodies and studies by the desmoglein (Dsg) 1 and desmoglein (Dsg) 3 ELISA and immunoblot method gave negative reactions. CIIF serum studies revealed a diffuse finely granular cell surface complement fixing antibody pattern, generally associated with a worse prognosis (Figure 2B) [8]. She was treated with oral prednisone, doxycycline, and niacinamide for the bullous disease and had some initial response but presented again with a severe flare of her skin disease along with a low grade fever (101.5°F), chills, myalgias, and fatigue. Follow up serum studies revealed that the previously observed cell surface CIIF disappeared, but that there was a positive complement fixing ANA (Figure 2C). Indirect immunofluoresence, immunoblot and ELISA studies for Dsg 1 and Dsg 3 antibodies again yielded negative findings. Further workup and treatment for non-Hodgkin lymphoma was not performed because of patient's fragile health. The patient was subsequently hospitalized for pneumonia and later developed sepsis and expired one month later.
Discussion
Diagnostic criteria for PNP and PAMS have included polymorphous mucocutaneous eruptions, histological findings of suprabasilar acantholysis, interface lichenoid changes and keratinocyte necrosis, direct immunofluorescence findings of cell surface and in some cases basement membrane zone deposits, positive staining of rat bladder epithelium by indirect immunofluorescence, and findings of antibodies to various proteins of the plakin gene family and Dsg 1 and Dsg 3 [1, 9, 10]. A finely beaded complement fixing cell surface antibody pattern is associated with a poor prognosis [8]. This case is unusual because there were no mucosal lesions and only a single detectable autoantibody in the serum. The routine histological findings were those of PNP, however, the patient had no detectable serum antibodies to Dsg 1 and Dsg 3 or any of the other autoantigens typically associated with pemphigus, PNP or PAMS.
A review of 163 cases revealed that 84 percent of cases of PNP are associated with hematologic malignancies (non-Hodgkin lymphoma: 38.6%, chronic lymphocytic leukemia: 18.4%, Castleman's disease: 18.4%). Non-hematologic malignancies comprised 16 percent of all cases [11]. Although this case does not meet specific criteria of PNP or PAMS, the findings and clinical course suggest that this case fits the spectrum of neoplasia-induced autoimmunity, first associated with PNP. We now know that the name PNP can be misleading, because cases may not always be pemphigus-like [6, 12, 13]. We suggest that patients with bullous, lichenoid, or graft versus host disease-like changes with atypical direct immunofluorescence findings and a history of underlying neoplasia should be evaluated by indirect immunofluorescence, ELISA, immunoblot, and CIIF. Identifying patients with neoplasia-induced autoimmunity will help further our understanding of this disease and direct appropriate therapy.
References
1. Anhalt GJ, Kim SC, Stanley JR et al. Paraneoplastic pemphigus: an autoimmune mucocutaneous disease associated with neoplasia. N Engl J Med 1990; 323:1729-1735. [PubMed]2. Beutner EH, Chorzelski TP, Hale WL and Petrusewicz IH. Autoimmunity in concurrent myasthenia gravis and pemphigus erythematosus. JAMA 1968; 203;125-129. [PubMed]
3. Matsuoka LY, Wortsman J, Stanley JR. Epidermal autoantibodies in erythema multiforme. J Am Acad Dermatol 1989; 21:677- 680. [PubMed]
4. van der Waal RI, Pas HH, Anhalt GJ, Schulten EA, Jonkman MF, Nieboer C. Paraneoplastic pemphigus as the presenting symptom of a lymphoma of the tongue. Oral Oncol 1998; 34:567-570. [PubMed]
5. Krunic AL, Kokai D, Bacetic B, Kesic V, Nikolic MM, Petkovic S, Clark RE. Retroperitoneal round-cell liposarcoma associated with paraneoplastic pemphigus presenting as lichen planus pemphigoides-like eruption. Int J Dermatol 1997; 36:526-529. [PubMed]
6. Nguyen VT, Ndoye A, Bassler KD et al. Classification, clinical manifestations, and immunopathological mechanisms of the epithelial variant of paraneoplastic autoimmune multiorgan syndrome: a reappraisal of paraneoplastic pemphigus. Arch Dermatol 2001; 137:193-206. [PubMed]
7. Wadhera A, Maverakis E, Mitsiades N, Lara PN et al. Thymoma associated multiorgan autoimmunity: a graft versus host like disease. J Am Acad Dermatol. 2007; 57:683-9. [PubMed]
8. Beutner EH, Pelton S, Hashimoto T, Xu Y, Plunkett RW, Korman NJ, Helm TN, Jablonska S. A nonfatal case and 2 fatal cases of paraneoplastic pemphigus: can a complement indirect immunofluorescent test help to identify fatal "group A" paraneoplastic pemphigus cases? J Am Acad Dermatol 2002; 47:841-851. [PubMed]
9. Horn TD, Anhalt GJ. Histologic features of paraneoplastic pemphigus. Arch Dermatol 1992; 128(8):1091-1095. [PubMed]
10. Coelho S, Reis JP, Tellechea O, Figueiredo A. and Black M. Paraneoplastic pemphigus with clinical features of lichen planus associated with low-grade B cell lymphoma. Int J Dermatol 2005; 44:366-371. [PubMed]
11. Kaplan I, Hodak E, Ackerman L, Mimouni D, Anhalt GJ, Calderon S. Neoplasms associated with paraneoplastic pemphigus: a review with emphasis on non-hematologic malignancy and oral mucosal manifestations. Oral Oncol. 2004 Jul;40(6):553-62. [PubMed]
12. Helm TN, Camisa C, Valenzuela R. Perplexing parlance of paraneoplastic pemphigus. JAMA. 1992; 268(5):602. [PubMed]
13. Helm TN, Grover R, and Beutner E. Neoplasia induced autoimmunity encompasses a spectrum of changes. J Am Acad Dermatol. 2008 Apr; 58(4):713. [PubMed]
© 2009 Dermatology Online Journal